| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1367829 | Bioorganic & Medicinal Chemistry Letters | 2005 | 5 Pages |
Abstract
Several hydroxamic acid derivatives with a substituted phthalimide group as a linker and/or cap structure, prepared during structural development studies based on thalidomide, were found to have histone deacetylase (HDAC)-inhibitory activity. Structure–activity relationship studies indicated that nature of the substituent introduced at the phthalimide nitrogen atom, introduction of a hydroxamic acid structure, and distance between the N-hydroxyl group and the cap structure are important for HDAC-inhibitory activity.
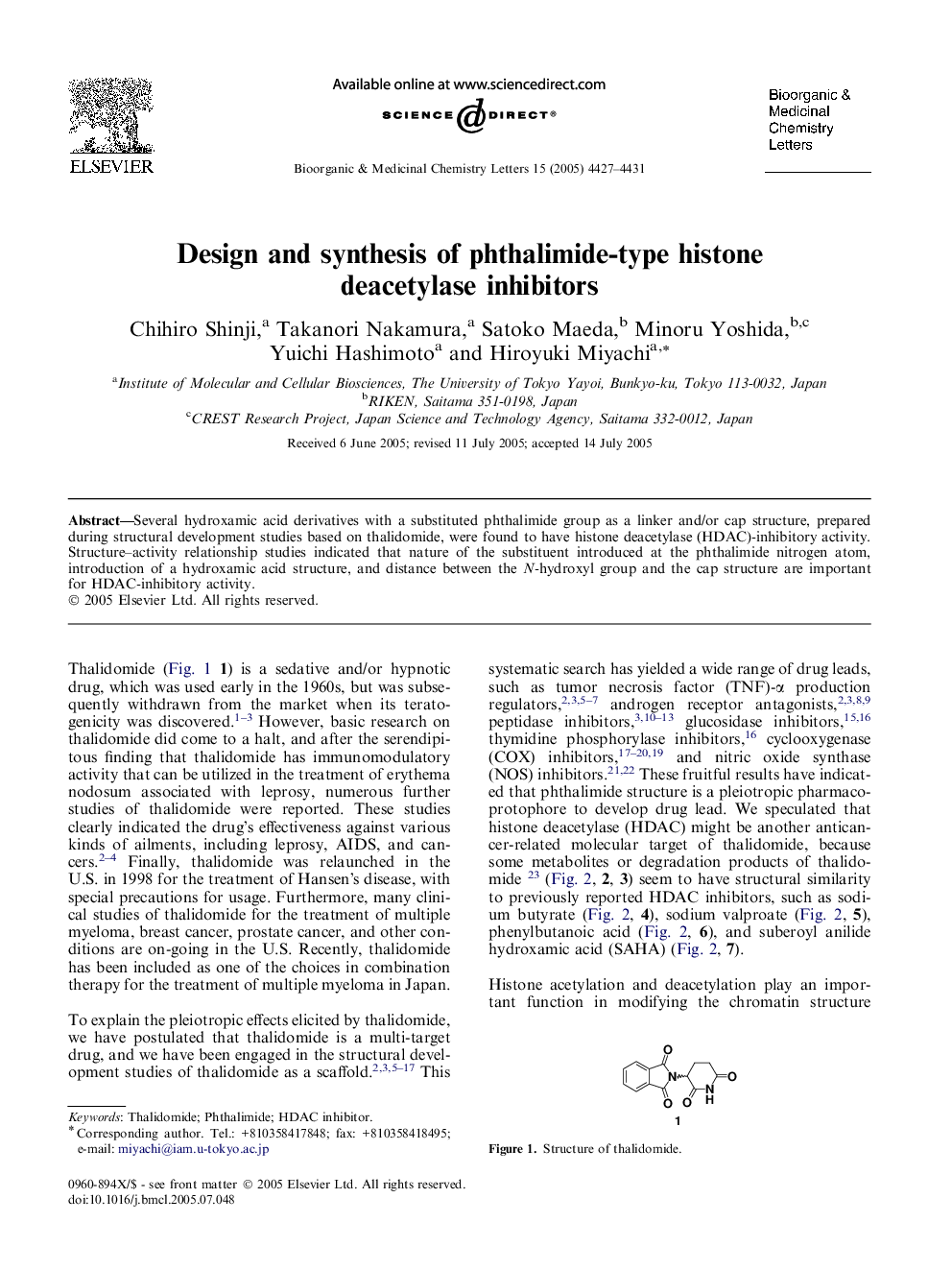
Graphical abstractThe design, synthesis, and HDAC-inhibitory activity of hydroxamic acids with a substituted phthalimide group are reported.Figure optionsDownload full-size imageDownload as PowerPoint slide
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Chihiro Shinji, Takanori Nakamura, Satoko Maeda, Minoru Yoshida, Yuichi Hashimoto, Hiroyuki Miyachi,