| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1367835 | Bioorganic & Medicinal Chemistry Letters | 2005 | 4 Pages |
Abstract
Substitution of the aryl sulfonamide moiety contained in MC4 agonist 1 with bicyclic heterocycles and aminotetralines produced compounds with MC4 activity. The heterocycles represent alternative privileged structures to that contained in 1. Compounds in which the polar group of the privileged structure was displayed in an endocyclic fashion were not as active as the parent agonist 1, while those with an exocyclic polar group afforded activity competitive with 1.
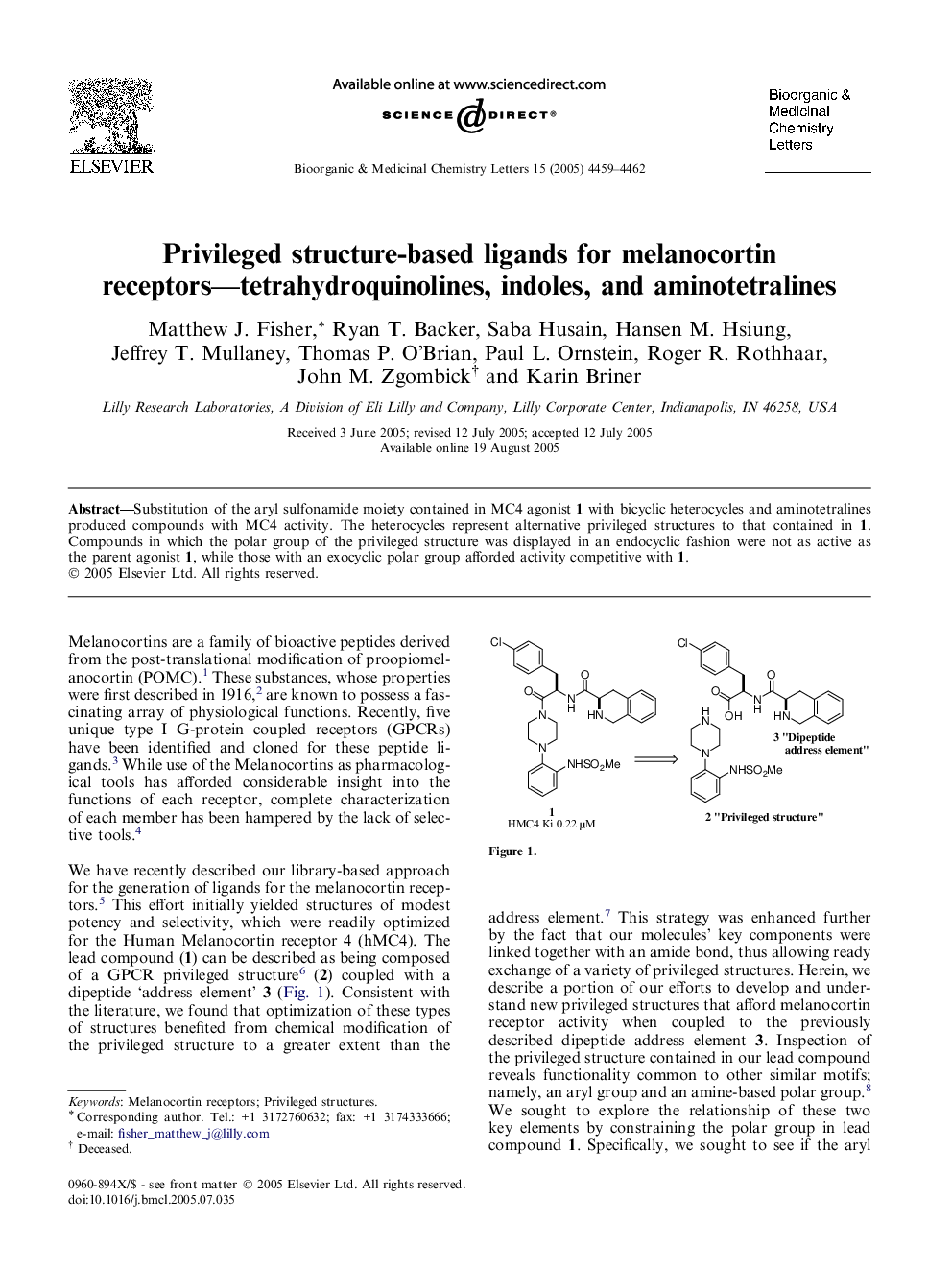
Graphical abstractTetrahydroquinolines, indoles, and aminotetralines provide useful privileged structures for the construction of ligands with affinity for melanocortin 4 receptors.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Matthew J. Fisher, Ryan T. Backer, Saba Husain, Hansen M. Hsiung, Jeffrey T. Mullaney, Thomas P. O’Brian, Paul L. Ornstein, Roger R. Rothhaar, John M. Zgombick, Karin Briner,