| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1367865 | Bioorganic & Medicinal Chemistry Letters | 2005 | 6 Pages |
Abstract
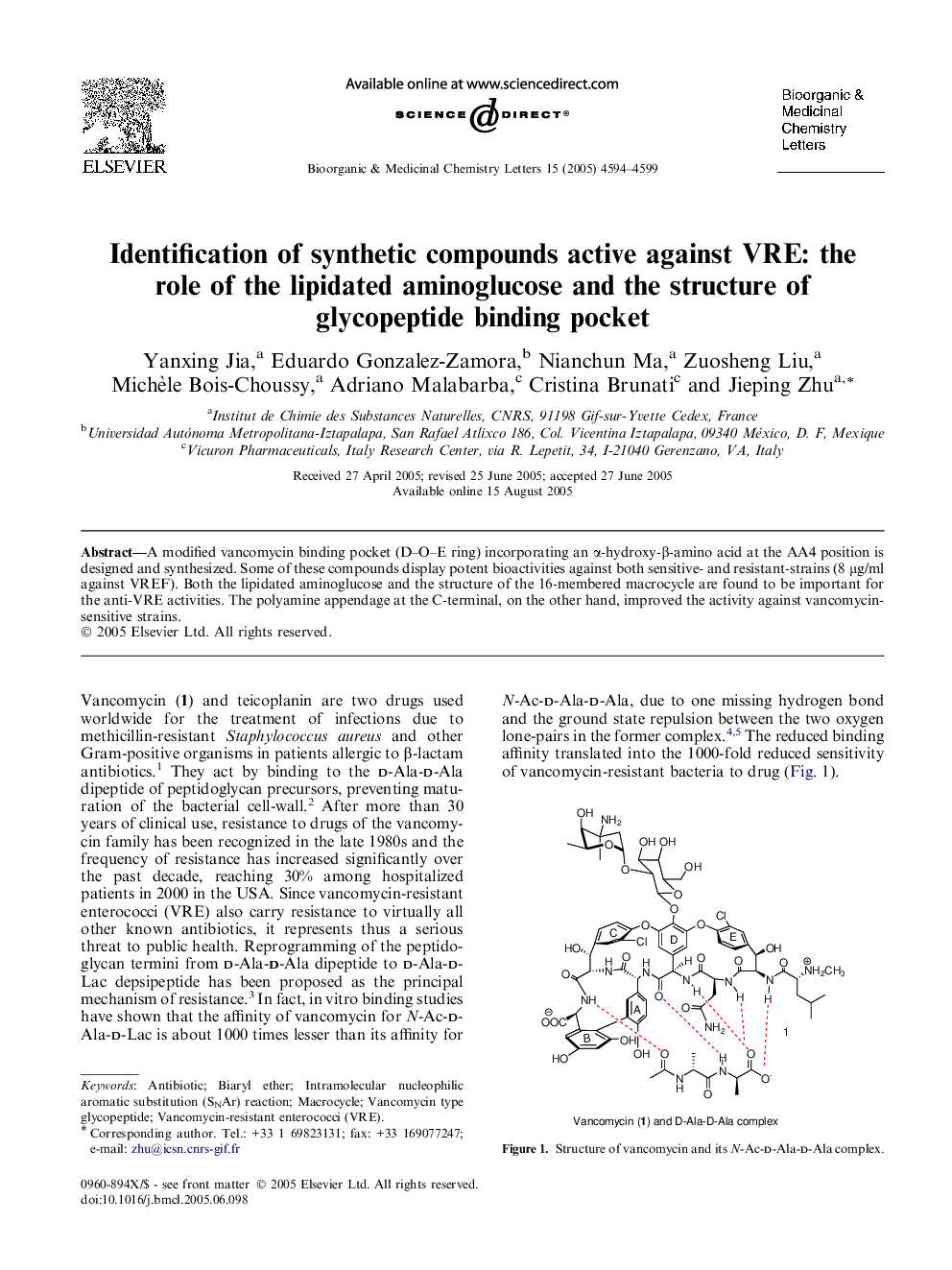
Synthesis of modified vancomycin binding pocket (D-O-E ring) incorporating a (R)- or (S)-configured secondary alcohol function at the AA4 position is described. The presence of both the lipidated aminoglucose (part A) and the structure of the 16-membered macrocycle (part B) are important for the observed activities of the modified vancomycin D-O-E ring against VRE. The polyamine appendage at the C-terminal (part C), on the other hand, improved the activity against vancomycin-sensitive strains.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Yanxing Jia, Eduardo Gonzalez-Zamora, Nianchun Ma, Zuosheng Liu, Michèle Bois-Choussy, Adriano Malabarba, Cristina Brunati, Jieping Zhu,