| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1369855 | Bioorganic & Medicinal Chemistry Letters | 2016 | 6 Pages |
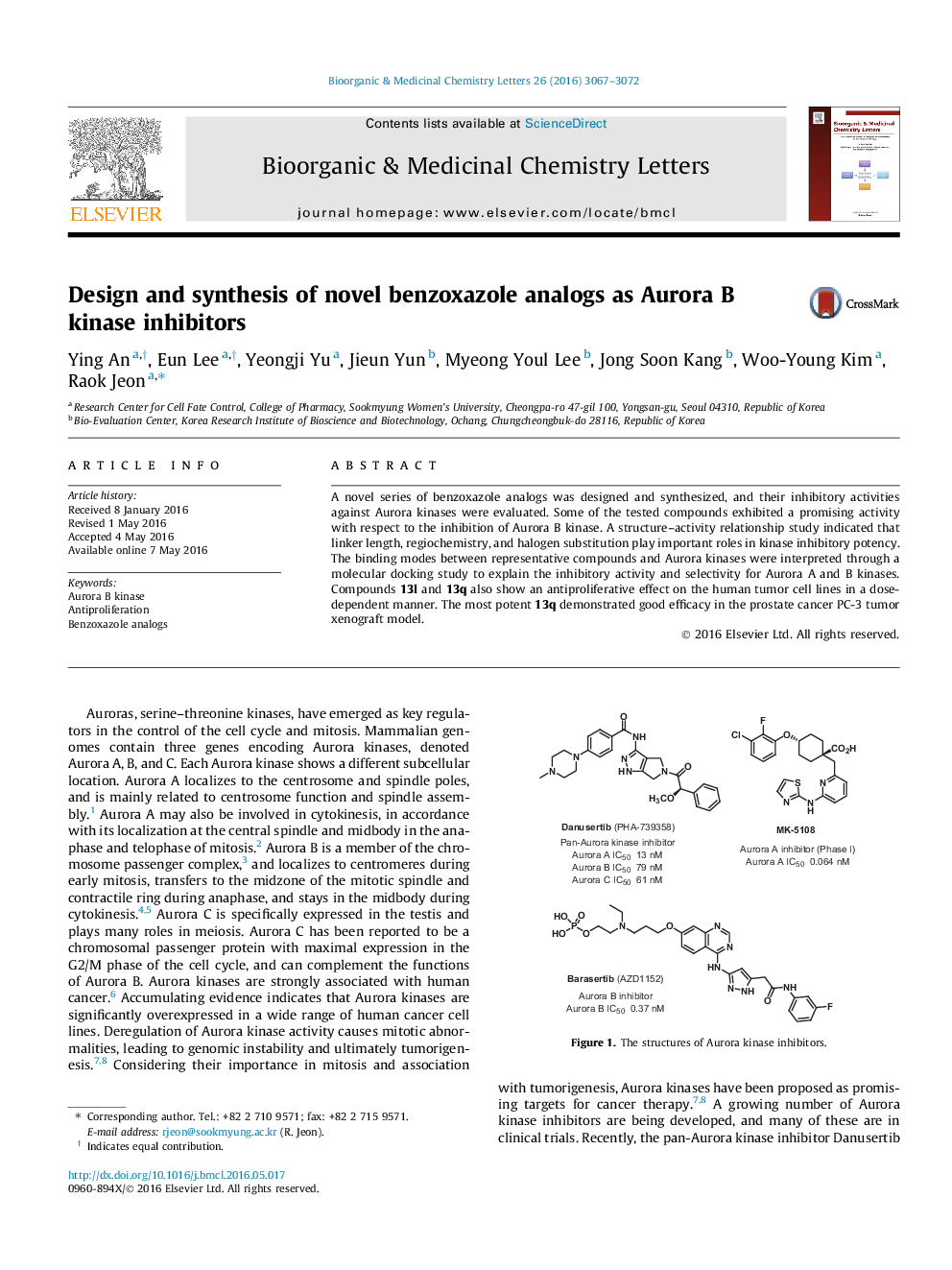
A novel series of benzoxazole analogs was designed and synthesized, and their inhibitory activities against Aurora kinases were evaluated. Some of the tested compounds exhibited a promising activity with respect to the inhibition of Aurora B kinase. A structure–activity relationship study indicated that linker length, regiochemistry, and halogen substitution play important roles in kinase inhibitory potency. The binding modes between representative compounds and Aurora kinases were interpreted through a molecular docking study to explain the inhibitory activity and selectivity for Aurora A and B kinases. Compounds 13l and 13q also show an antiproliferative effect on the human tumor cell lines in a dose-dependent manner. The most potent 13q demonstrated good efficacy in the prostate cancer PC-3 tumor xenograft model.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide