| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1369980 | Bioorganic & Medicinal Chemistry Letters | 2012 | 6 Pages |
Abstract
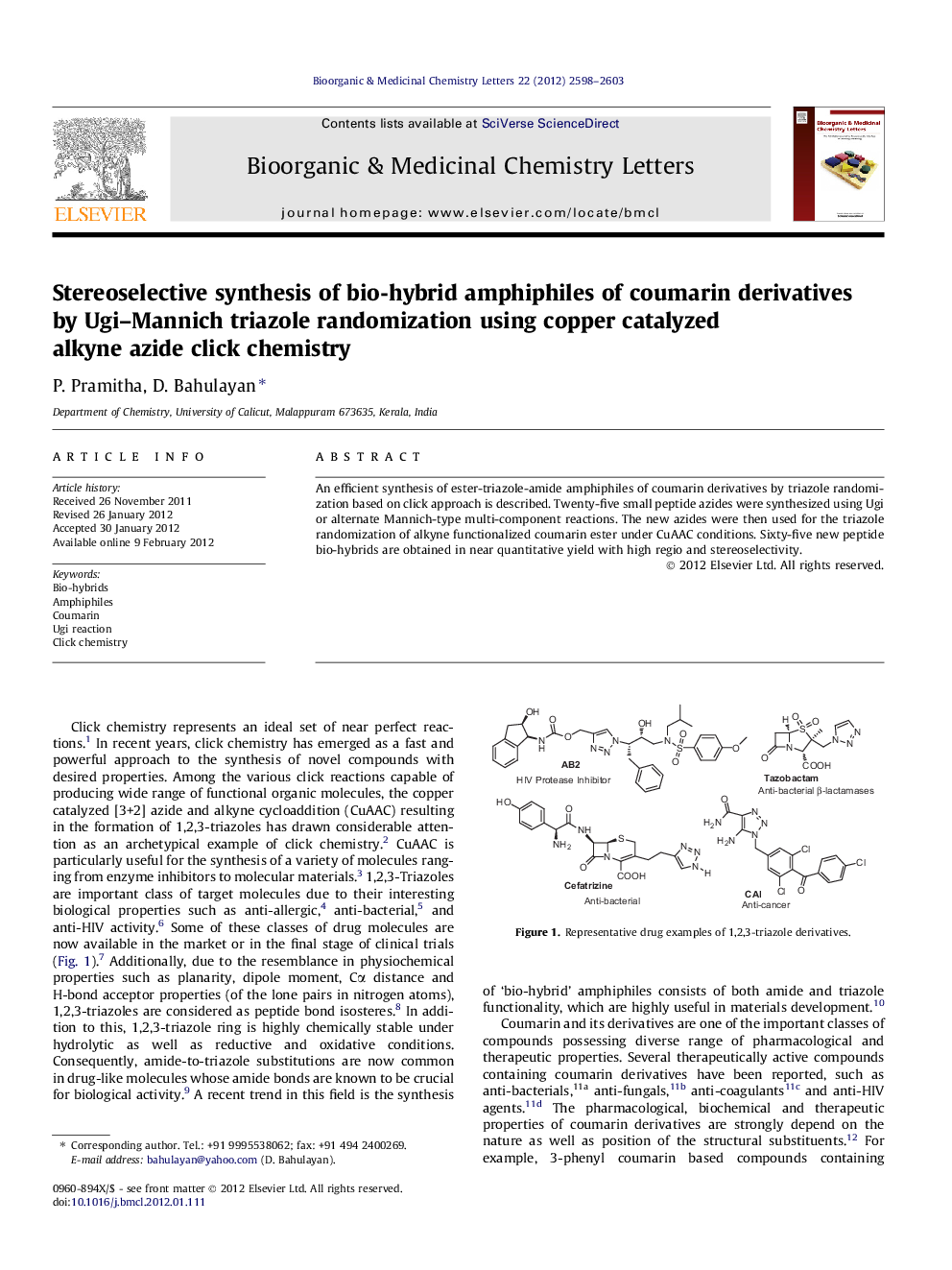
An efficient synthesis of ester-triazole-amide amphiphiles of coumarin derivatives by triazole randomization based on click approach is described. Twenty-five small peptide azides were synthesized using Ugi or alternate Mannich-type multi-component reactions. The new azides were then used for the triazole randomization of alkyne functionalized coumarin ester under CuAAC conditions. Sixty-five new peptide bio-hybrids are obtained in near quantitative yield with high regio and stereoselectivity.
Graphical abstractSixty five examples.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
P. Pramitha, D. Bahulayan,