| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1370907 | Bioorganic & Medicinal Chemistry Letters | 2011 | 4 Pages |
Abstract
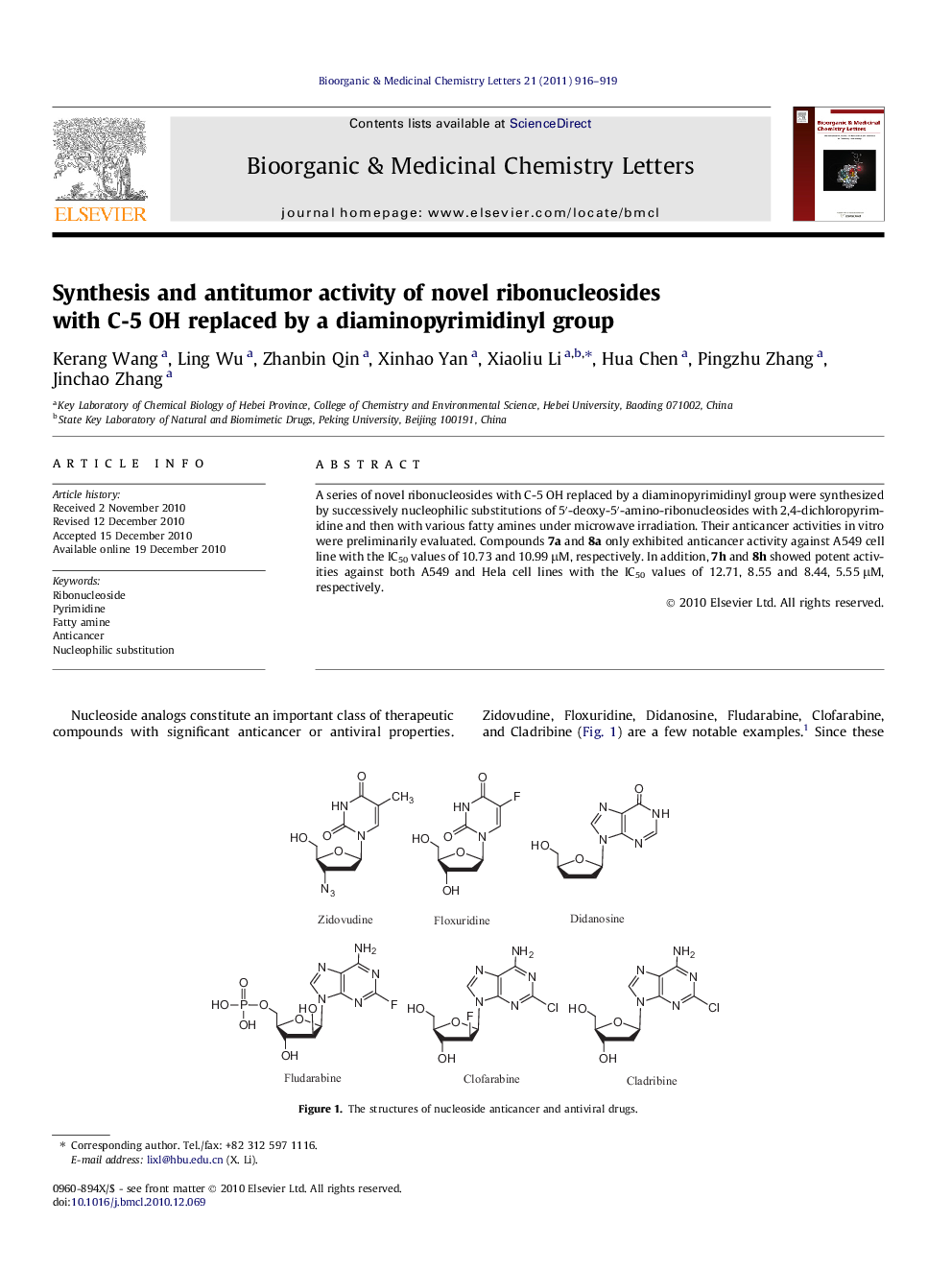
A series of novel ribonucleosides with C-5 OH replaced by a diaminopyrimidinyl group were synthesized by successively nucleophilic substitutions of 5′-deoxy-5′-amino-ribonucleosides with 2,4-dichloropyrimidine and then with various fatty amines under microwave irradiation. Their anticancer activities in vitro were preliminarily evaluated. Compounds 7a and 8a only exhibited anticancer activity against A549 cell line with the IC50 values of 10.73 and 10.99 μM, respectively. In addition, 7h and 8h showed potent activities against both A549 and Hela cell lines with the IC50 values of 12.71, 8.55 and 8.44, 5.55 μM, respectively.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Kerang Wang, Ling Wu, Zhanbin Qin, Xinhao Yan, Xiaoliu Li, Hua Chen, Pingzhu Zhang, Jinchao Zhang,