| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1371041 | Bioorganic & Medicinal Chemistry Letters | 2015 | 5 Pages |
Abstract
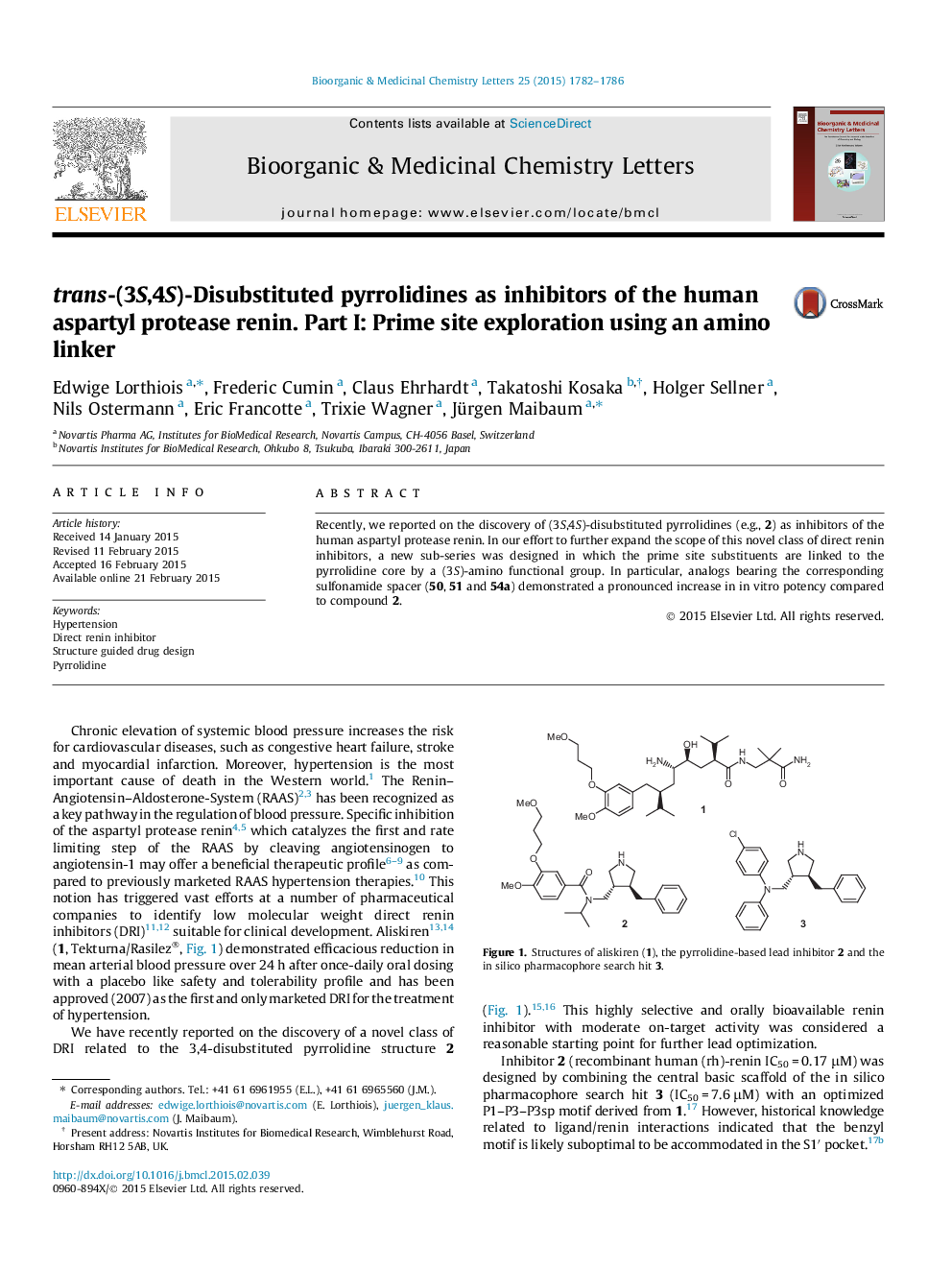
Recently, we reported on the discovery of (3S,4S)-disubstituted pyrrolidines (e.g., 2) as inhibitors of the human aspartyl protease renin. In our effort to further expand the scope of this novel class of direct renin inhibitors, a new sub-series was designed in which the prime site substituents are linked to the pyrrolidine core by a (3S)-amino functional group. In particular, analogs bearing the corresponding sulfonamide spacer (50, 51 and 54a) demonstrated a pronounced increase in in vitro potency compared to compound 2.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Edwige Lorthiois, Frederic Cumin, Claus Ehrhardt, Takatoshi Kosaka, Holger Sellner, Nils Ostermann, Eric Francotte, Trixie Wagner, Jürgen Maibaum,