| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1371362 | Bioorganic & Medicinal Chemistry Letters | 2010 | 6 Pages |
Abstract
We have optimized a novel series of potent p38 MAP kinase inhibitors based on an α-ketoamide scaffold through structure based design that due to their extended molecular architecture bind, in addition to the ATP site, to an allosteric pocket. In vitro ADME, in vivo PK and efficacy studies show these compounds to have drug-like characteristics and have resulted in the nomination of a development candidate which is currently in phase II clinical trials for the oral treatment of inflammatory conditions.
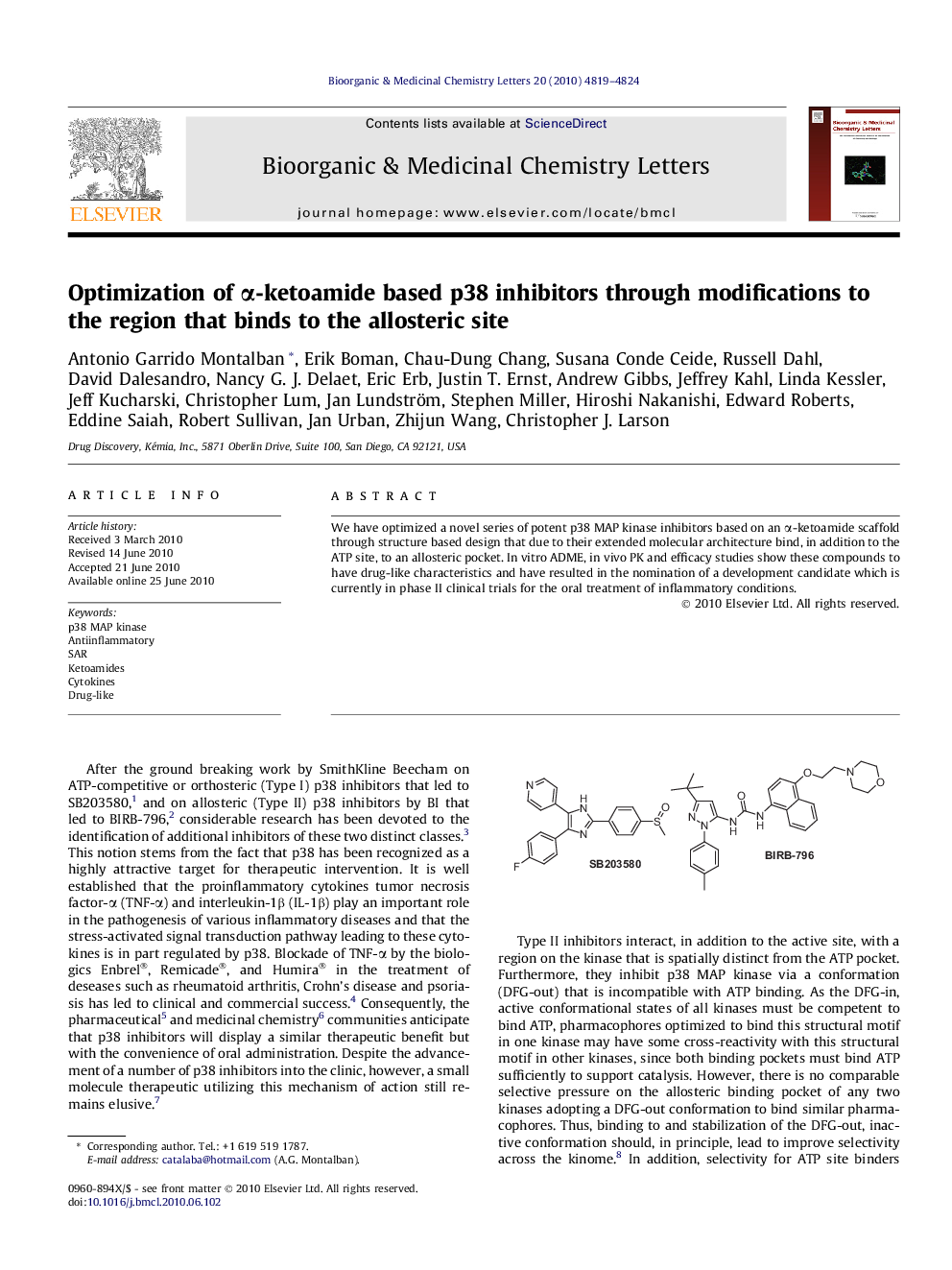
Graphical abstractThe design and synthesis of novel α-ketoamide based p38 inhibitors is reported.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Antonio Garrido Montalban, Erik Boman, Chau-Dung Chang, Susana Conde Ceide, Russell Dahl, David Dalesandro, Nancy G.J. Delaet, Eric Erb, Justin T. Ernst, Andrew Gibbs, Jeffrey Kahl, Linda Kessler, Jeff Kucharski, Christopher Lum, Jan Lundström,