| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1371656 | Bioorganic & Medicinal Chemistry Letters | 2010 | 6 Pages |
Abstract
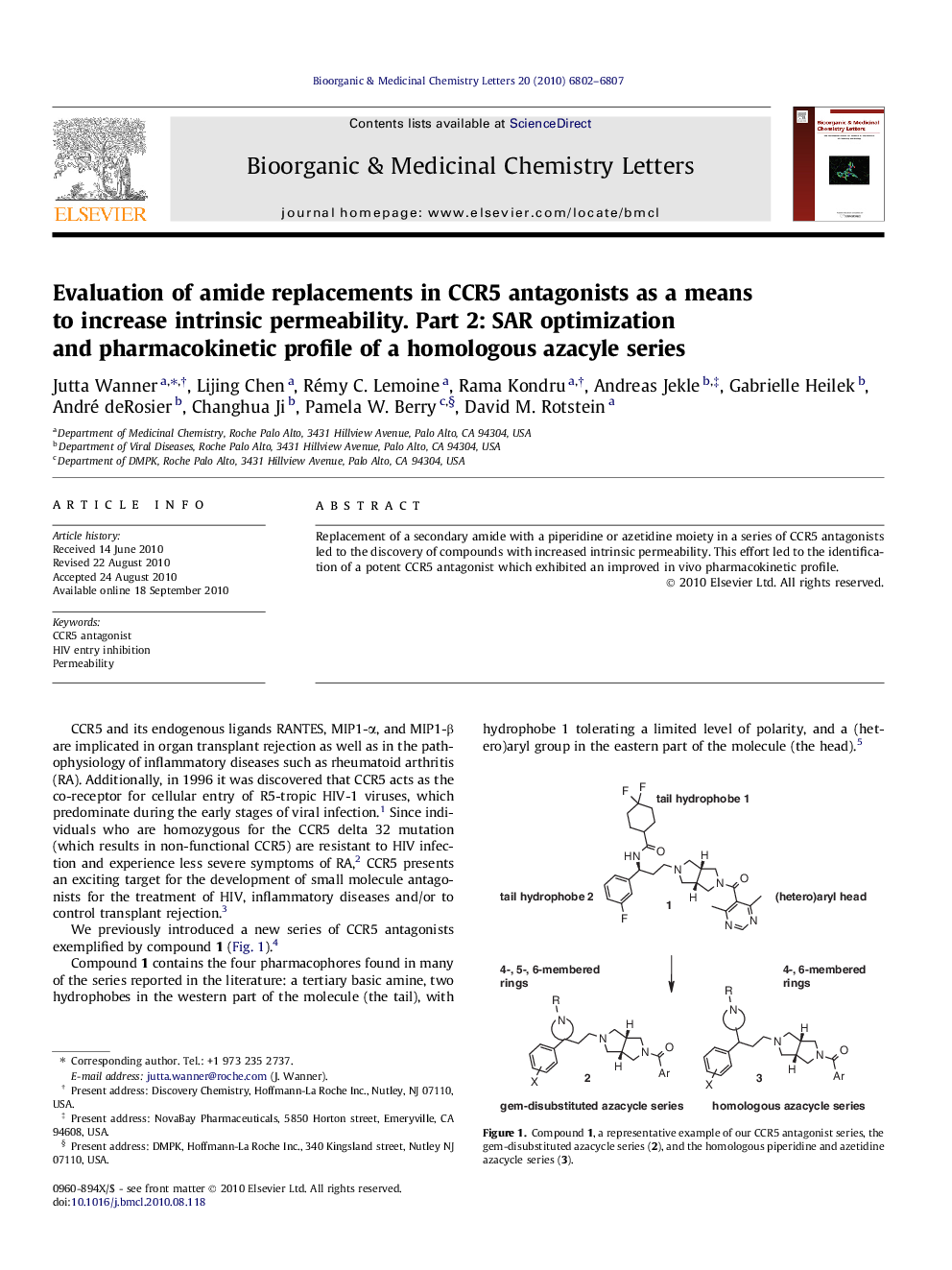
Replacement of a secondary amide with a piperidine or azetidine moiety in a series of CCR5 antagonists led to the discovery of compounds with increased intrinsic permeability. This effort led to the identification of a potent CCR5 antagonist which exhibited an improved in vivo pharmacokinetic profile.
Graphical abstractIn our CCR5 program, piperidine and azetidine amide replacements as means to improve intrinsic permeability were evaluated. This led to new series of potent CCR5 antagonists, with improved PK behavior.Figure optionsDownload full-size imageDownload as PowerPoint slide
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Jutta Wanner, Lijing Chen, Rémy C. Lemoine, Rama Kondru, Andreas Jekle, Gabrielle Heilek, André deRosier, Changhua Ji, Pamela W. Berry, David M. Rotstein,