| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1372549 | Bioorganic & Medicinal Chemistry Letters | 2011 | 4 Pages |
Abstract
The synthesis of novel β-functionalized derivatives of the clinically used photosensitizer Temoporfin has been achieved by nucleophilic addition reactions to a corresponding diketo chlorin. The β-substituted dihydroxychlorin products exhibit a strong absorption in the red spectral region, a high singlet oxygen quantum yield, and were found to be highly effective in in vitro assays against HT-29 tumor cells.
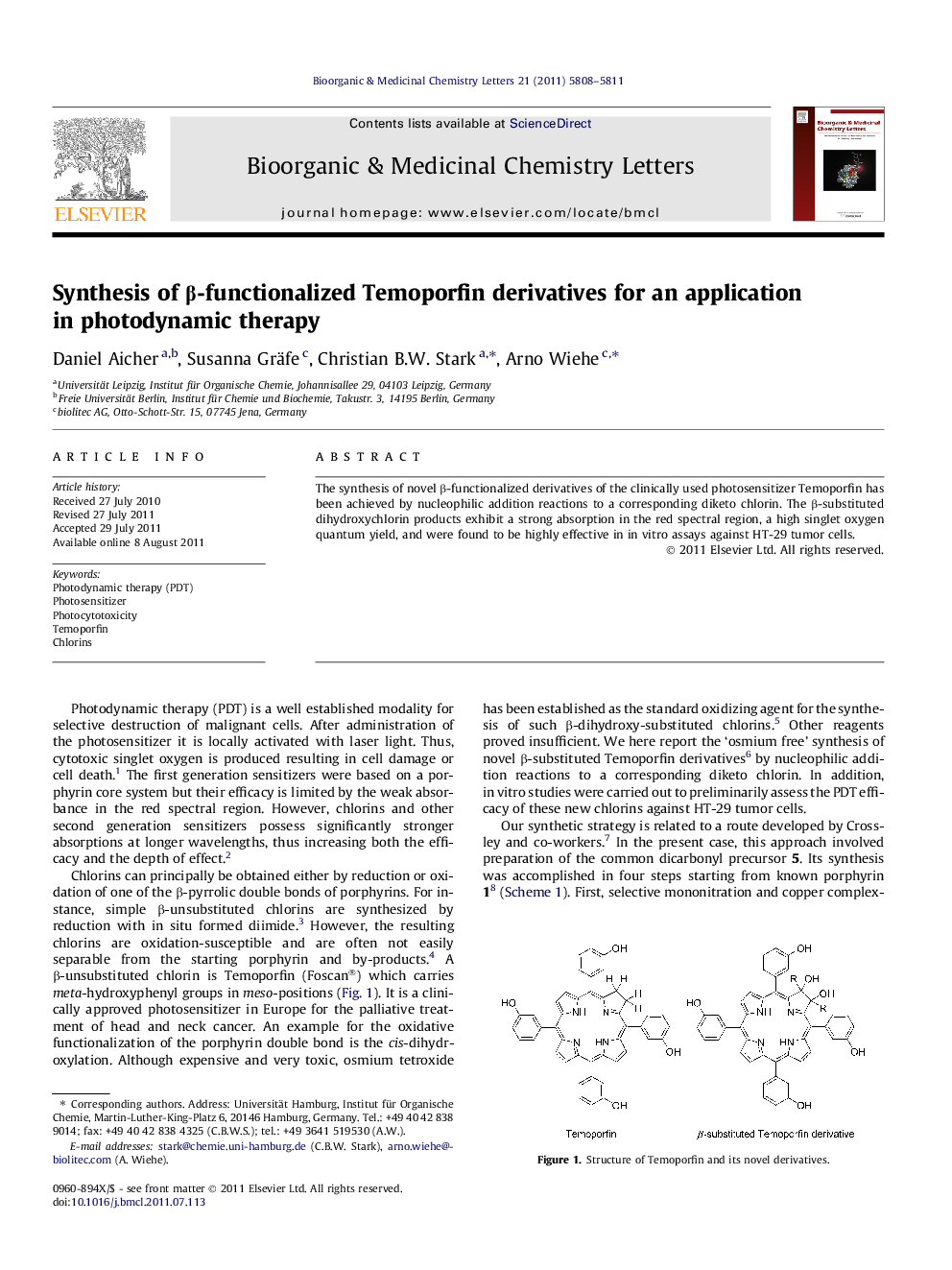
Graphical abstractβ-Substituted derivatives of the clinically used photosensitizer Temoporfin have been prepared via a novel synthetic route. Compared to Temoporfin itself these chlorins are more densely functionalized and possess a higher chemical stability.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Daniel Aicher, Susanna Gräfe, Christian B.W. Stark, Arno Wiehe,