| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1372915 | Bioorganic & Medicinal Chemistry Letters | 2011 | 5 Pages |
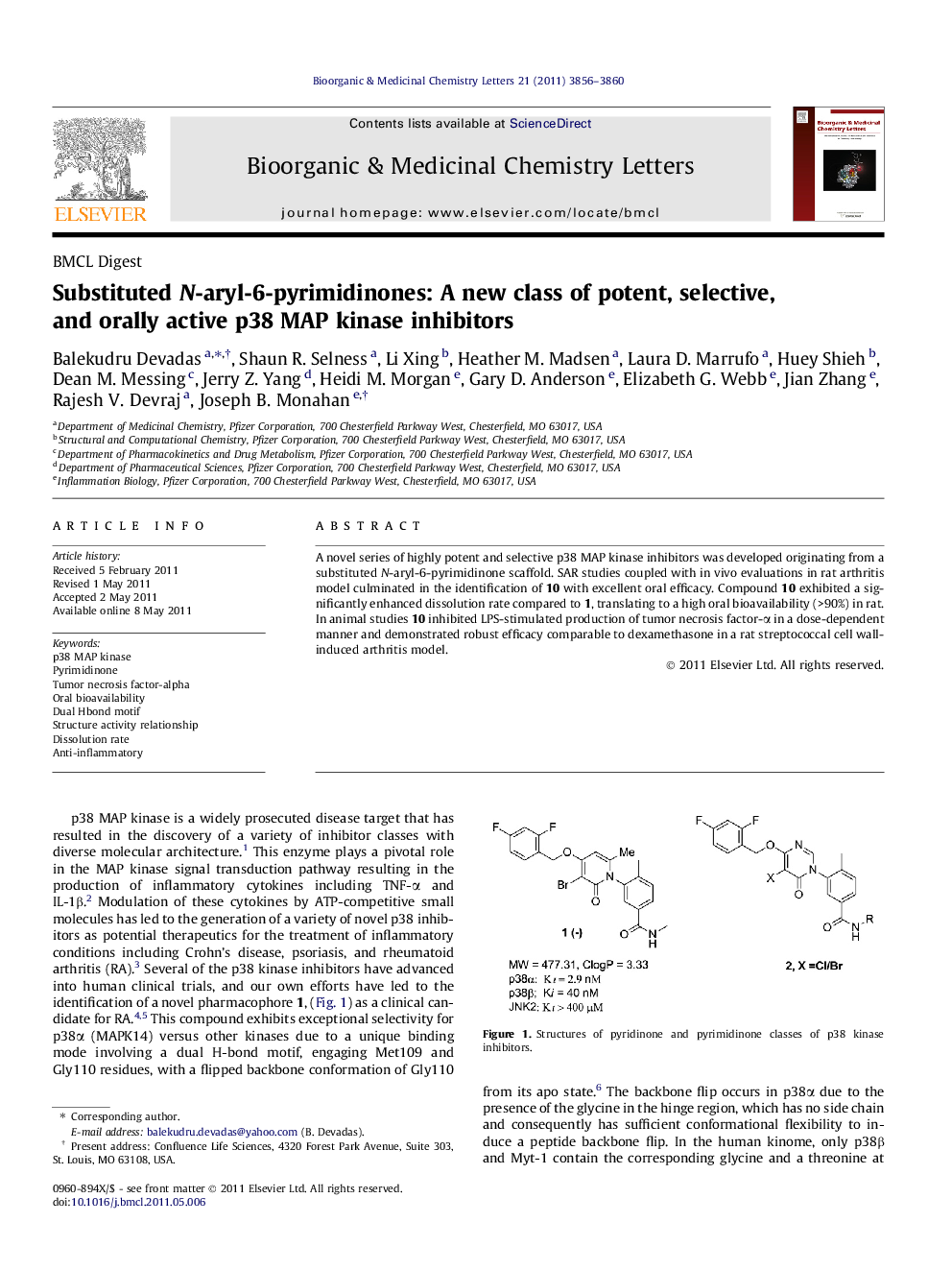
A novel series of highly potent and selective p38 MAP kinase inhibitors was developed originating from a substituted N-aryl-6-pyrimidinone scaffold. SAR studies coupled with in vivo evaluations in rat arthritis model culminated in the identification of 10 with excellent oral efficacy. Compound 10 exhibited a significantly enhanced dissolution rate compared to 1, translating to a high oral bioavailability (>90%) in rat. In animal studies 10 inhibited LPS-stimulated production of tumor necrosis factor-α in a dose-dependent manner and demonstrated robust efficacy comparable to dexamethasone in a rat streptococcal cell wall-induced arthritis model.
Graphical abstractA novel series of highly potent and selective p38 MAP kinase inhibitors was developed originating from a substituted N-aryl-6-pyrimidinone scaffold. SAR studies of these analogues coupled with in vivo evaluations in a rat RA disease model, led to the selection of 10 as the clinical candidate for the treatment of inflammatory diseases.Figure optionsDownload full-size imageDownload as PowerPoint slide