| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1372932 | Bioorganic & Medicinal Chemistry Letters | 2011 | 4 Pages |
Abstract
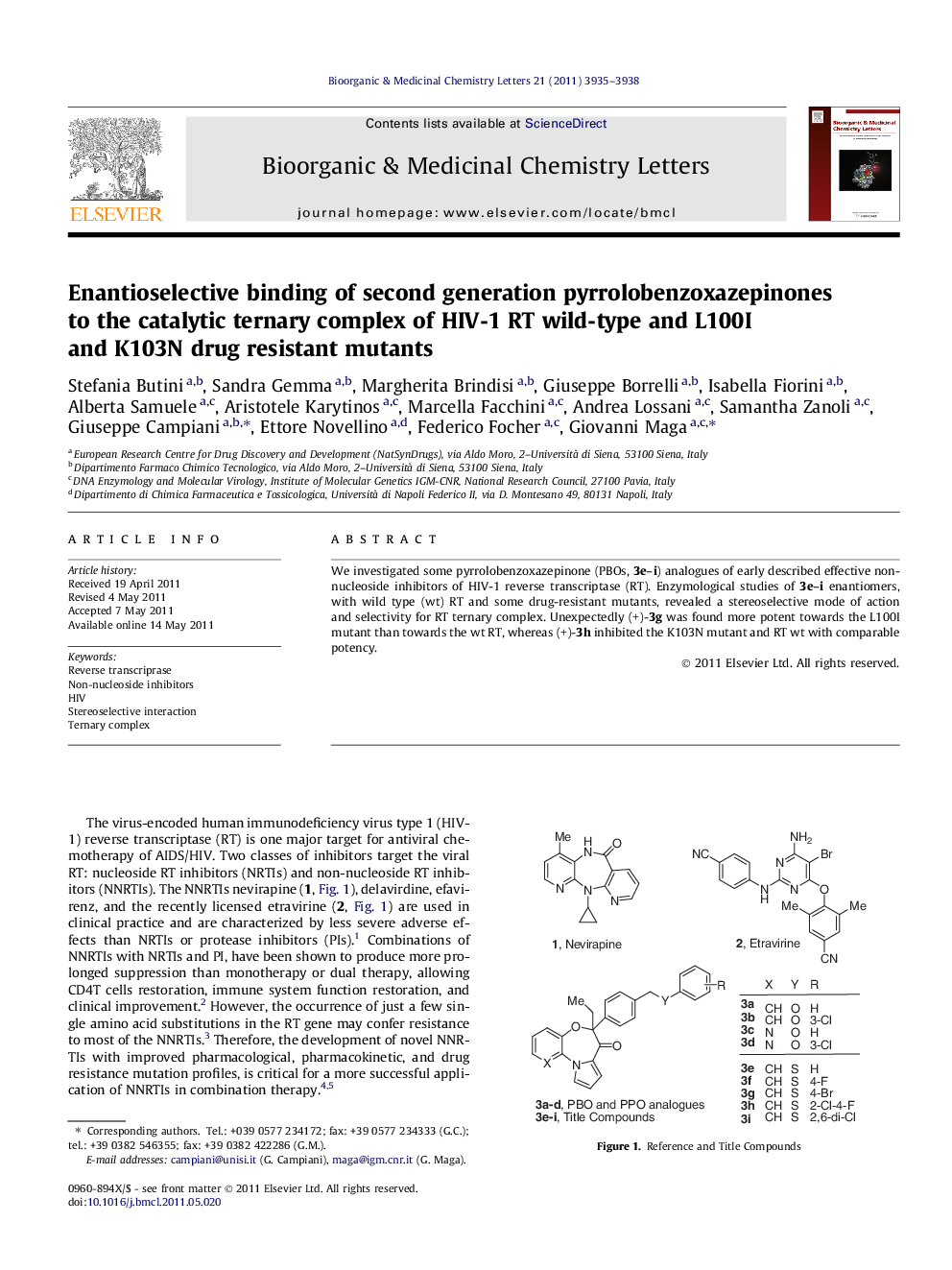
We investigated some pyrrolobenzoxazepinone (PBOs, 3e–i) analogues of early described effective non-nucleoside inhibitors of HIV-1 reverse transcriptase (RT). Enzymological studies of 3e–i enantiomers, with wild type (wt) RT and some drug-resistant mutants, revealed a stereoselective mode of action and selectivity for RT ternary complex. Unexpectedly (+)-3g was found more potent towards the L100I mutant than towards the wt RT, whereas (+)-3h inhibited the K103N mutant and RT wt with comparable potency.
Graphical abstractThe enantioselective inhibitory activity of benzoxazepinones against the HIV-1 RT ternary complex is reported.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Stefania Butini, Sandra Gemma, Margherita Brindisi, Giuseppe Borrelli, Isabella Fiorini, Alberta Samuele, Aristotele Karytinos, Marcella Facchini, Andrea Lossani, Samantha Zanoli, Giuseppe Campiani, Ettore Novellino, Federico Focher, Giovanni Maga,