| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1372941 | Bioorganic & Medicinal Chemistry Letters | 2011 | 6 Pages |
Abstract
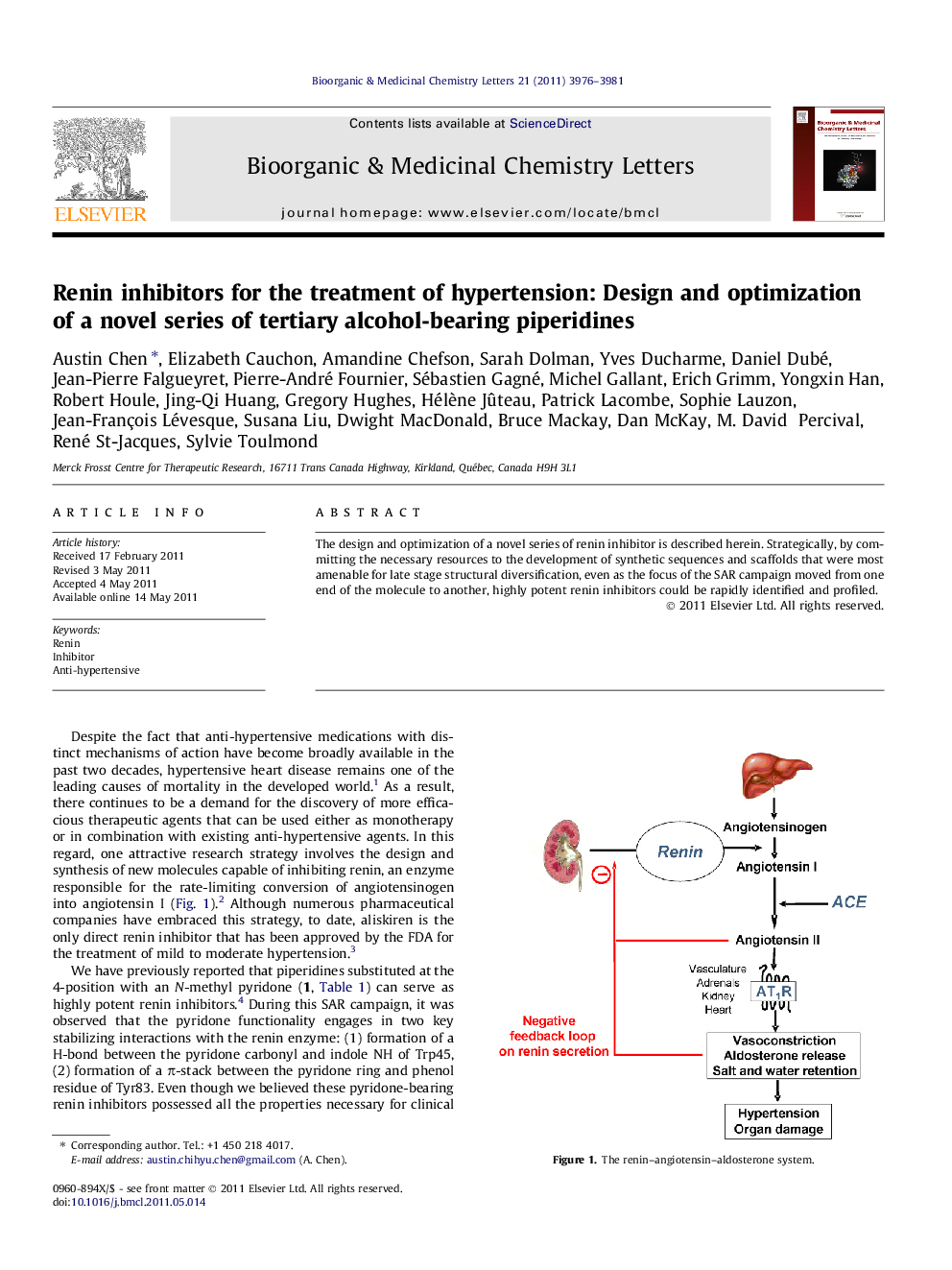
The design and optimization of a novel series of renin inhibitor is described herein. Strategically, by committing the necessary resources to the development of synthetic sequences and scaffolds that were most amenable for late stage structural diversification, even as the focus of the SAR campaign moved from one end of the molecule to another, highly potent renin inhibitors could be rapidly identified and profiled.
Graphical abstractThe discovery of a novel series of potent, orally-bioavailable renin inhibitor through a rational, structure-guided truncation and optimization campaign.Figure optionsDownload full-size imageDownload as PowerPoint slide
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Austin Chen, Elizabeth Cauchon, Amandine Chefson, Sarah Dolman, Yves Ducharme, Daniel Dubé, Jean-Pierre Falgueyret, Pierre-André Fournier, Sébastien Gagné, Michel Gallant, Erich Grimm, Yongxin Han, Robert Houle, Jing-Qi Huang, Gregory Hughes,