| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1373364 | Bioorganic & Medicinal Chemistry Letters | 2010 | 4 Pages |
Abstract
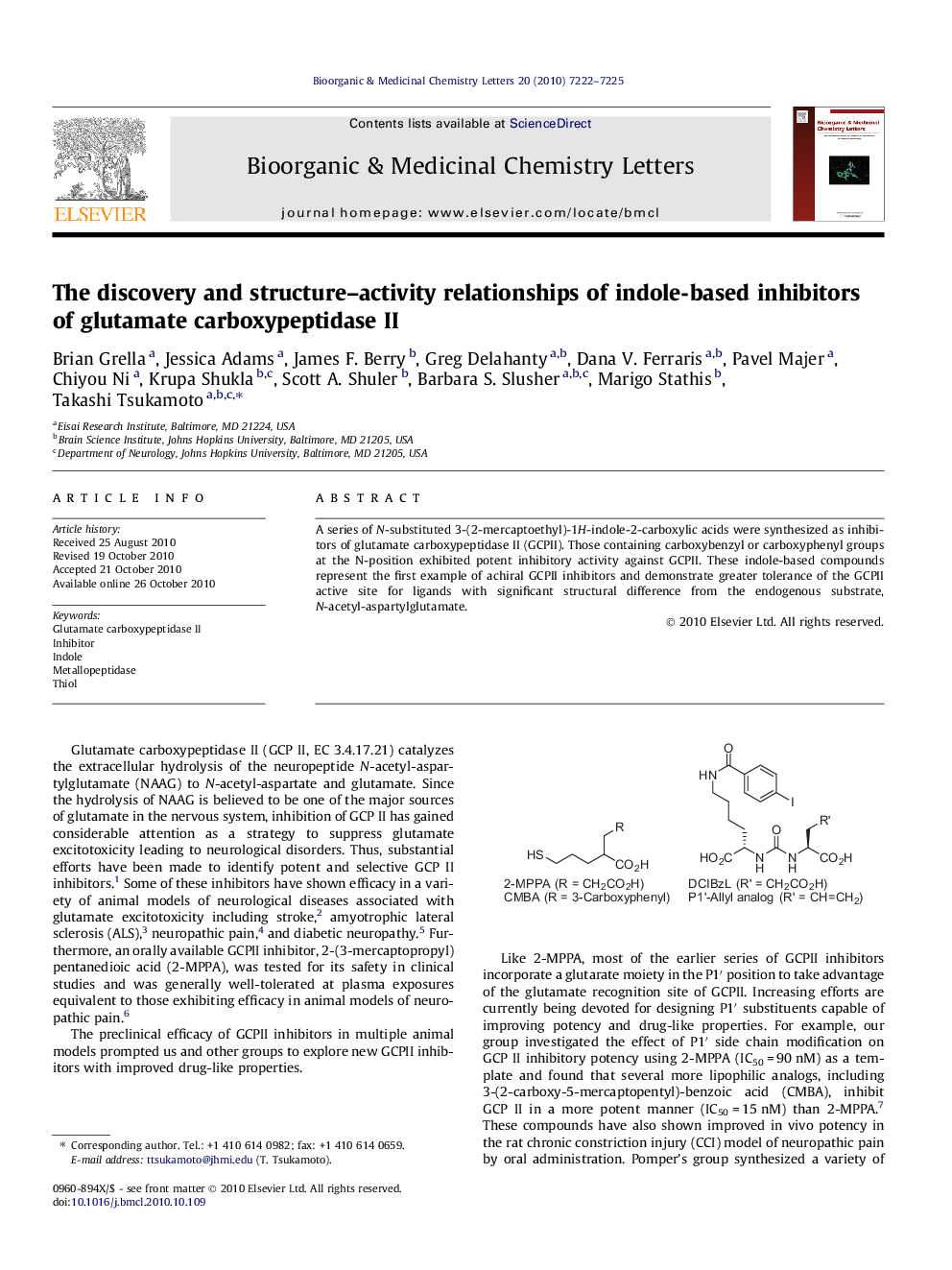
A series of N-substituted 3-(2-mercaptoethyl)-1H-indole-2-carboxylic acids were synthesized as inhibitors of glutamate carboxypeptidase II (GCPII). Those containing carboxybenzyl or carboxyphenyl groups at the N-position exhibited potent inhibitory activity against GCPII. These indole-based compounds represent the first example of achiral GCPII inhibitors and demonstrate greater tolerance of the GCPII active site for ligands with significant structural difference from the endogenous substrate, N-acetyl-aspartylglutamate.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Brian Grella, Jessica Adams, James F. Berry, Greg Delahanty, Dana V. Ferraris, Pavel Majer, Chiyou Ni, Krupa Shukla, Scott A. Shuler, Barbara S. Slusher, Marigo Stathis, Takashi Tsukamoto,