| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1374551 | Bioorganic & Medicinal Chemistry Letters | 2010 | 4 Pages |
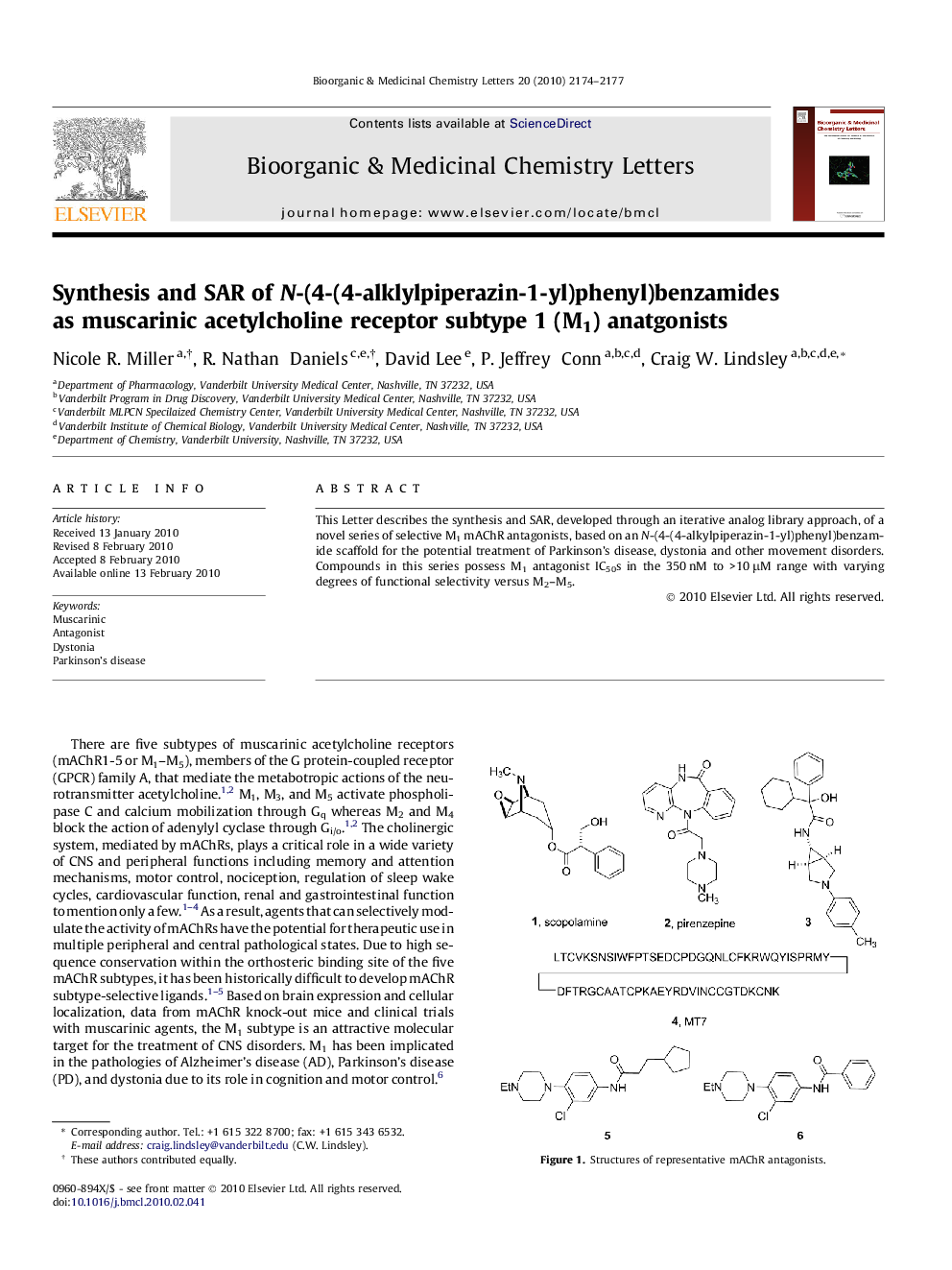
This Letter describes the synthesis and SAR, developed through an iterative analog library approach, of a novel series of selective M1 mAChR antagonists, based on an N-(4-(4-alkylpiperazin-1-yl)phenyl)benzamide scaffold for the potential treatment of Parkinson’s disease, dystonia and other movement disorders. Compounds in this series possess M1 antagonist IC50s in the 350 nM to >10 μM range with varying degrees of functional selectivity versus M2–M5.
Graphical abstractThis Letter describes the synthesis and SAR, developed through an iterative analog library approach, of a novel series of selective M1 mAChR antagonists, based on an N-(4-(4-alkylpiperazin-1-yl)phenyl)benzamide scaffold for the potential treatment of Parkinson’s disease, dystonia and other movement disorders. Compounds in this series possess M1 antagonist IC50s in the 350 nM to >10 μM range with varying degrees of functional selectivity versus M2–M5.Figure optionsDownload full-size imageDownload as PowerPoint slide