| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1376007 | Bioorganic & Medicinal Chemistry Letters | 2005 | 4 Pages |
Abstract
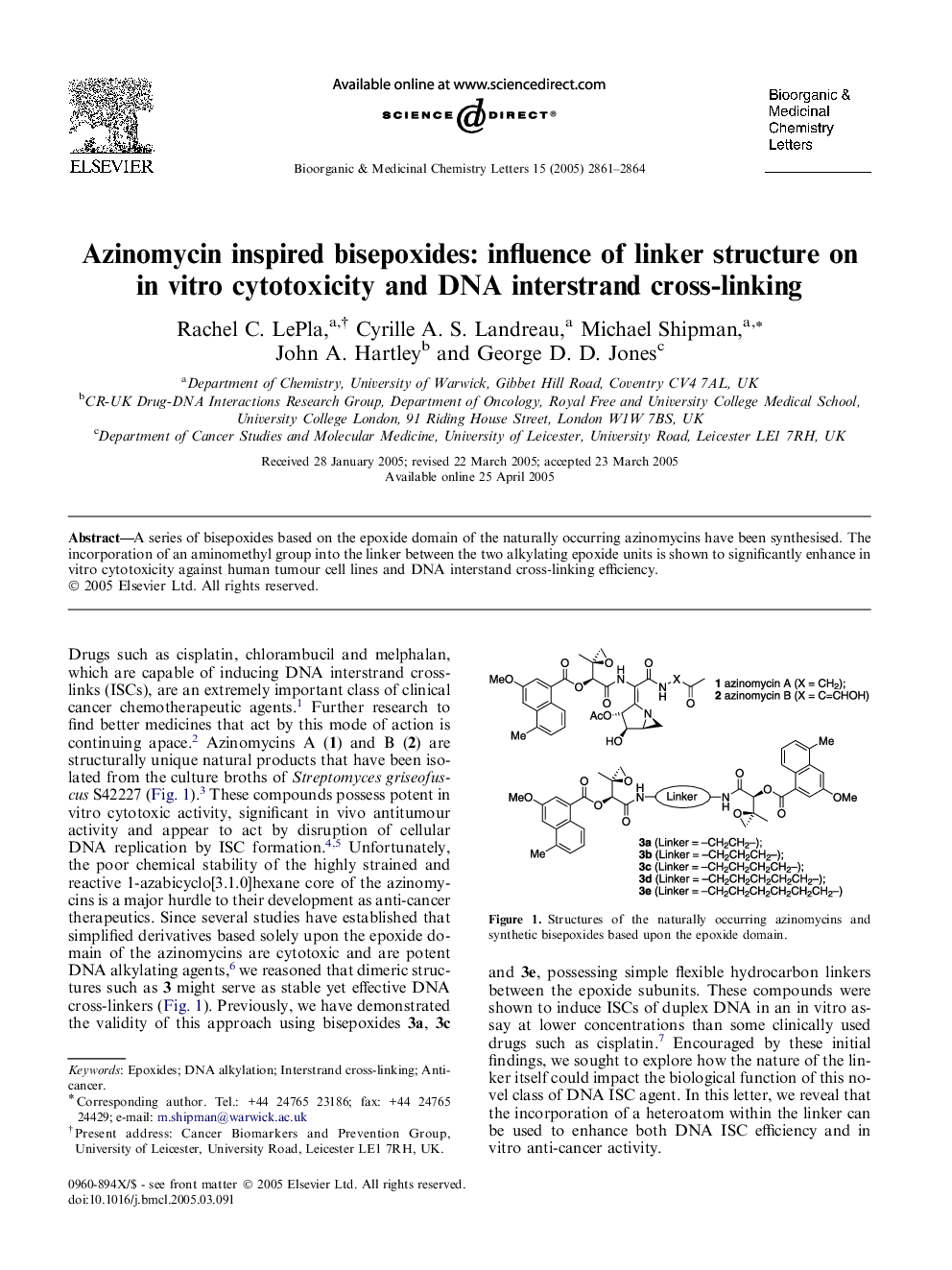
A series of bisepoxides based on the epoxide domain of the naturally occurring azinomycins have been synthesised. The incorporation of an aminomethyl group into the linker between the two alkylating epoxide units is shown to significantly enhance in vitro cytotoxicity against human tumour cell lines and DNA interstand cross-linking efficiency.
Graphical abstractIn in vitro assays, it is shown that changing X = CH2 into X = NMe results in 7–9-fold increase in DNA interstrand cross-linking activity and a 3–10-fold increase in cytotoxicity against human tumour cell lines.Figure optionsDownload full-size imageDownload as PowerPoint slide
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Rachel C. LePla, Cyrille A.S. Landreau, Michael Shipman, John A. Hartley, George D.D. Jones,