| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1376339 | Bioorganic & Medicinal Chemistry Letters | 2008 | 4 Pages |
Abstract
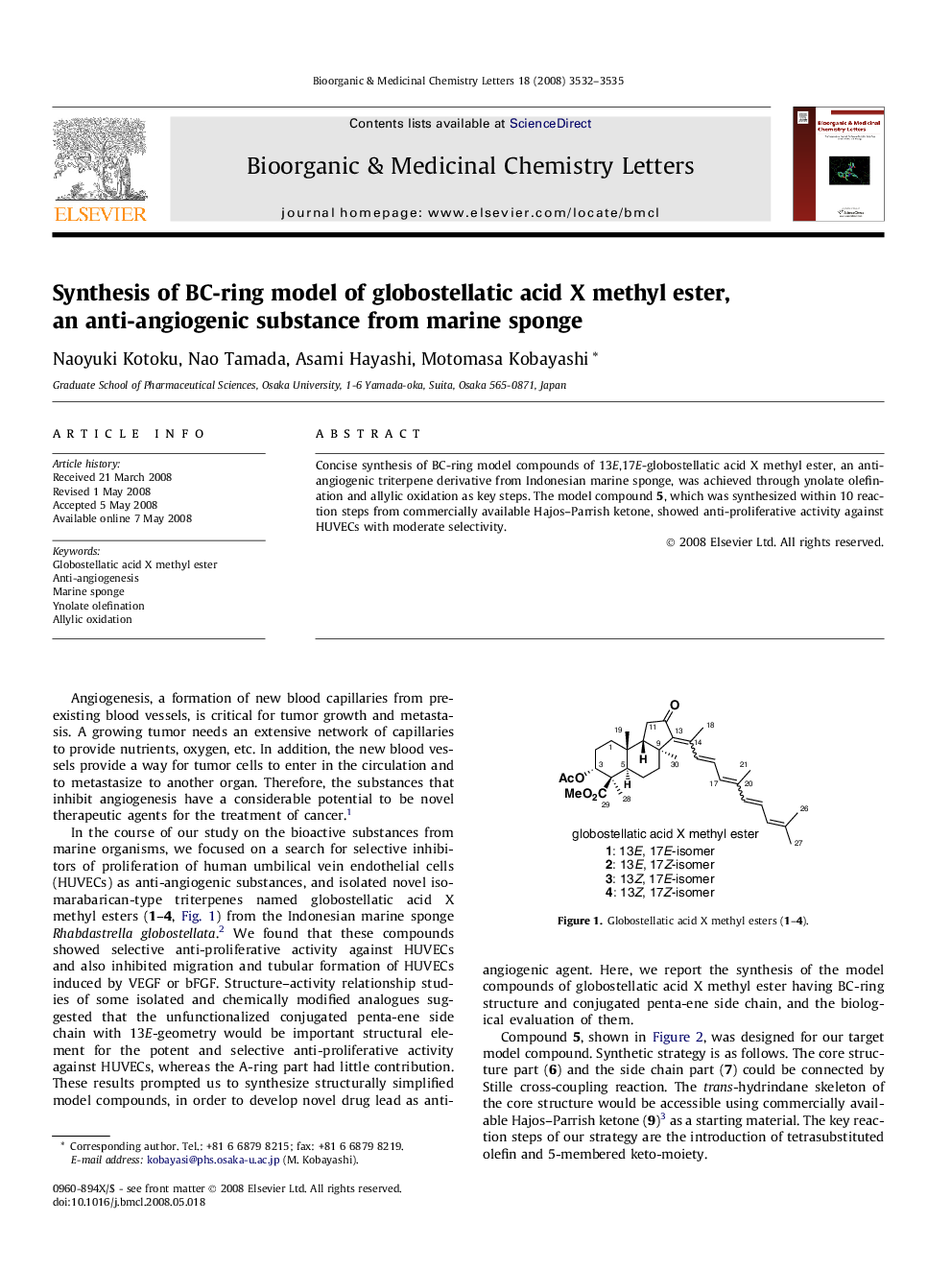
Concise synthesis of BC-ring model compounds of 13E,17E-globostellatic acid X methyl ester, an anti-angiogenic triterpene derivative from Indonesian marine sponge, was achieved through ynolate olefination and allylic oxidation as key steps. The model compound 5, which was synthesized within 10 reaction steps from commercially available Hajos–Parrish ketone, showed anti-proliferative activity against HUVECs with moderate selectivity.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Naoyuki Kotoku, Nao Tamada, Asami Hayashi, Motomasa Kobayashi,