| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1376486 | Bioorganic & Medicinal Chemistry Letters | 2006 | 4 Pages |
Abstract
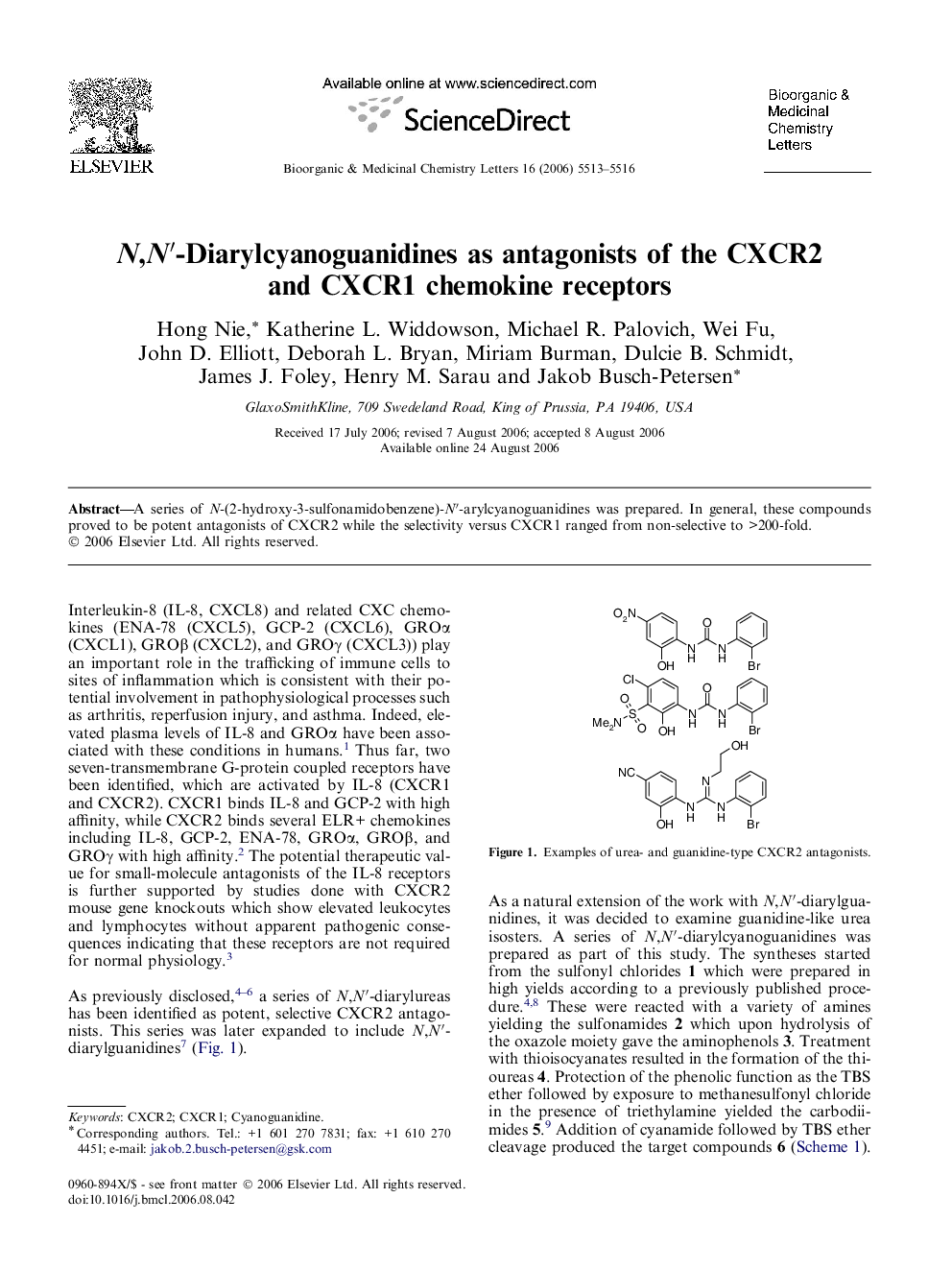
A series of N-(2-hydroxy-3-sulfonamidobenzene)-N′-arylcyanoguanidines was prepared. In general, these compounds proved to be potent antagonists of CXCR2 while the selectivity versus CXCR1 ranged from non-selective to >200-fold.
Graphical abstractA series of N-(2-hydroxy-3-sulfonamidobenzene)-N′-arylcyanoguanidines was prepared. In general, these compounds proved to be potent antagonists of CXCR2 while the selectivity versus CXCR1 ranged from non-selective to >200-fold.Figure optionsDownload full-size imageDownload as PowerPoint slide
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Hong Nie, Katherine L. Widdowson, Michael R. Palovich, Wei Fu, John D. Elliott, Deborah L. Bryan, Miriam Burman, Dulcie B. Schmidt, James J. Foley, Henry M. Sarau, Jakob Busch-Petersen,