| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1376601 | Bioorganic & Medicinal Chemistry Letters | 2008 | 4 Pages |
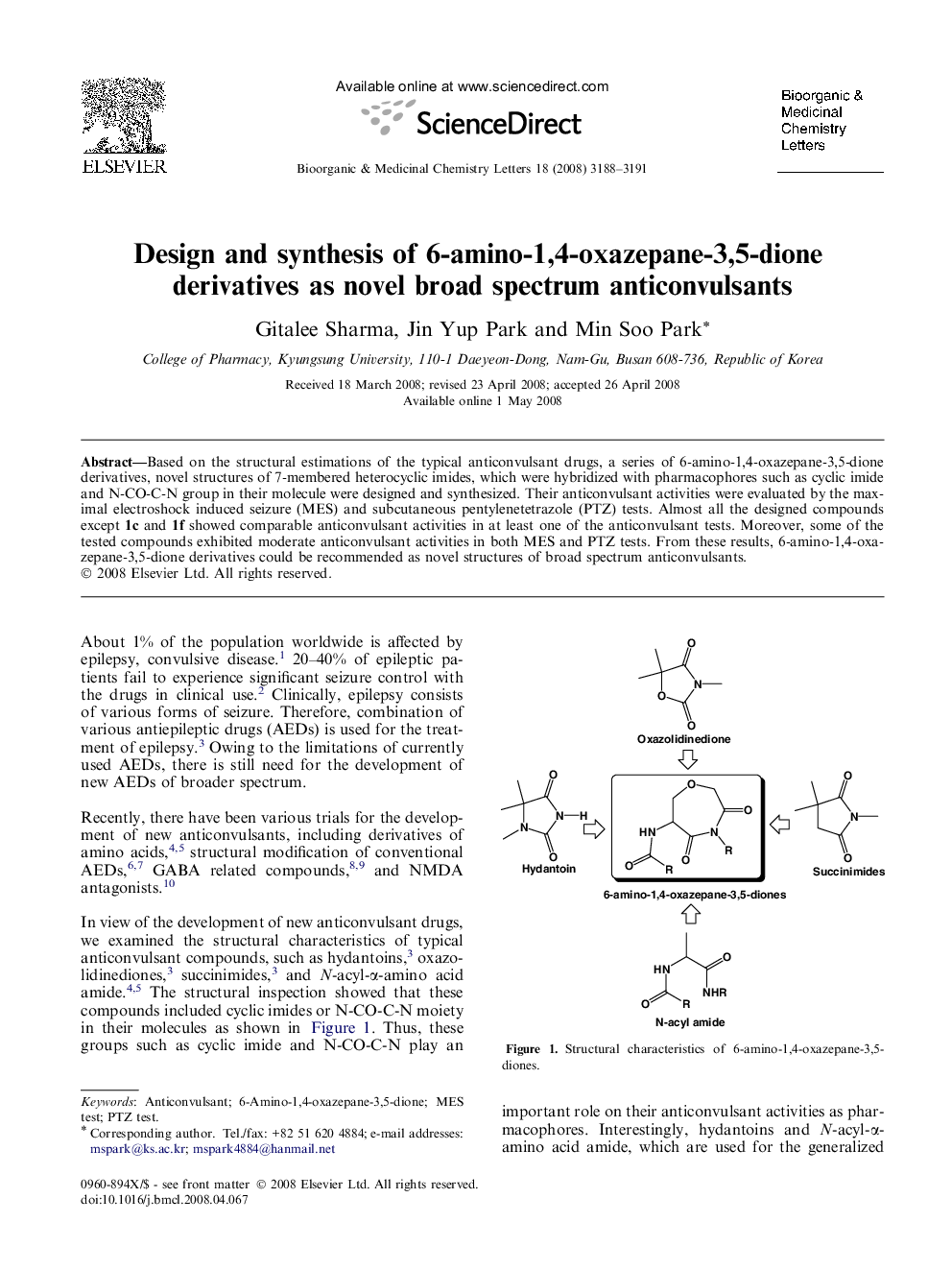
Based on the structural estimations of the typical anticonvulsant drugs, a series of 6-amino-1,4-oxazepane-3,5-dione derivatives, novel structures of 7-membered heterocyclic imides, which were hybridized with pharmacophores such as cyclic imide and N-CO-C-N group in their molecule were designed and synthesized. Their anticonvulsant activities were evaluated by the maximal electroshock induced seizure (MES) and subcutaneous pentylenetetrazole (PTZ) tests. Almost all the designed compounds except 1c and 1f showed comparable anticonvulsant activities in at least one of the anticonvulsant tests. Moreover, some of the tested compounds exhibited moderate anticonvulsant activities in both MES and PTZ tests. From these results, 6-amino-1,4-oxazepane-3,5-dione derivatives could be recommended as novel structures of broad spectrum anticonvulsants.
Graphical abstractA series of 6-amino-1,4-oxazepane-3,5-dione derivatives, which are 7-membered heterocyclic imides and have N-CO-C-N group in their structures, were designed, synthesized, and their anticonvulsant activities were evaluated by both the MES and PTZ tests.Figure optionsDownload full-size imageDownload as PowerPoint slide