| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1376634 | Bioorganic & Medicinal Chemistry Letters | 2008 | 5 Pages |
Overexpression of cIAP1 correlates with resistance to radiotherapy and chemotherapy in various cancers. Recently, we reported that a class of bestatin ester analogs represented by MeBS (2) destabilized and promoted the degradation of cIAP1 through auto-ubiquitination, and thereby sensitized cancer cells to apoptosis. Herein, we present chemical evidence that bestatin ester analogs directly interact with the cIAP1-BIR3 domain by means of fluorescence polarization assay and photoaffinity labeling assay using fluorescent probes.
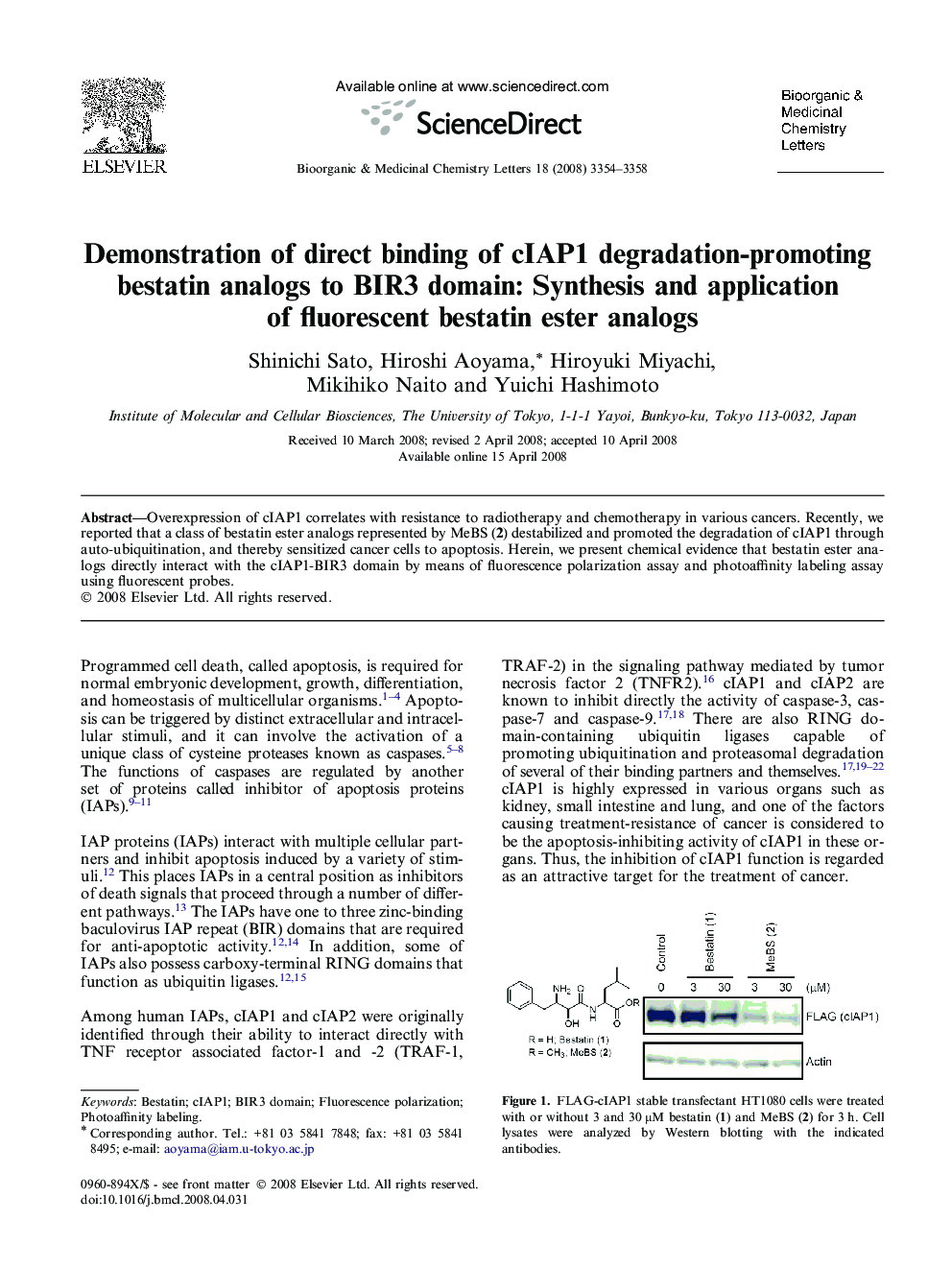
Graphical abstractFluorescent bestatin ester analogs 3 and 4 were designed and synthesized. Direct binding of cIAP1-BIR3 domain protein and these probes was observed in fluorescence polarization assay and photoaffinity labeling assay.Figure optionsDownload full-size imageDownload as PowerPoint slide