| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1376761 | Bioorganic & Medicinal Chemistry Letters | 2006 | 5 Pages |
Abstract
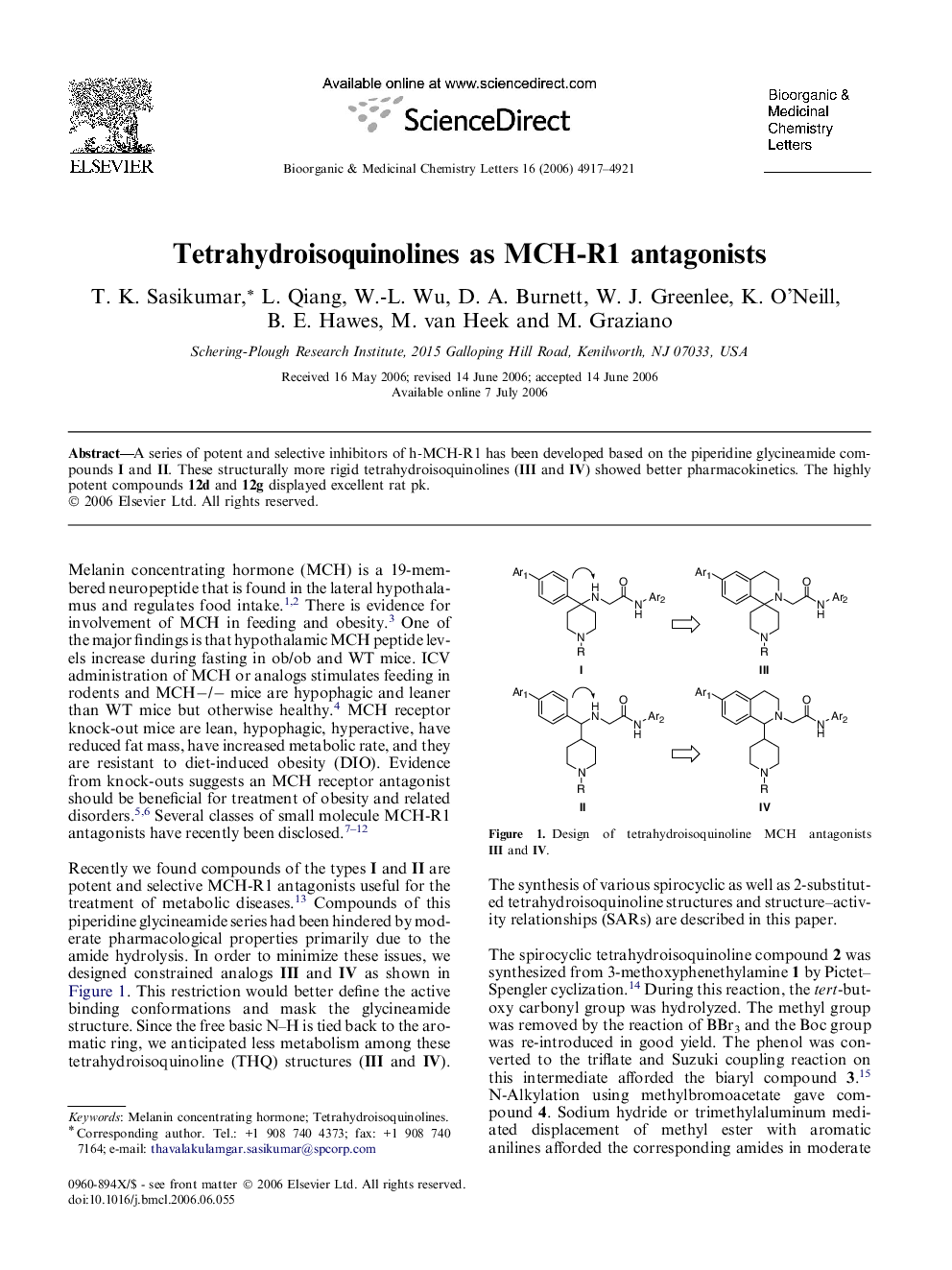
A series of potent and selective inhibitors of h-MCH-R1 has been developed based on the piperidine glycineamide compounds I and II. These structurally more rigid tetrahydroisoquinolines (III and IV) showed better pharmacokinetics. The highly potent compounds 12d and 12g displayed excellent rat pk.
Graphical abstractA series of potent and selective MCH-R1 antagonists have been discovered based on a piperidine glycineamide series.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
T.K. Sasikumar, L. Qiang, W.-L. Wu, D.A. Burnett, W.J. Greenlee, K. O’Neill, B.E. Hawes, M. van Heek, M. Graziano,