| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1377404 | Bioorganic & Medicinal Chemistry Letters | 2007 | 4 Pages |
Abstract
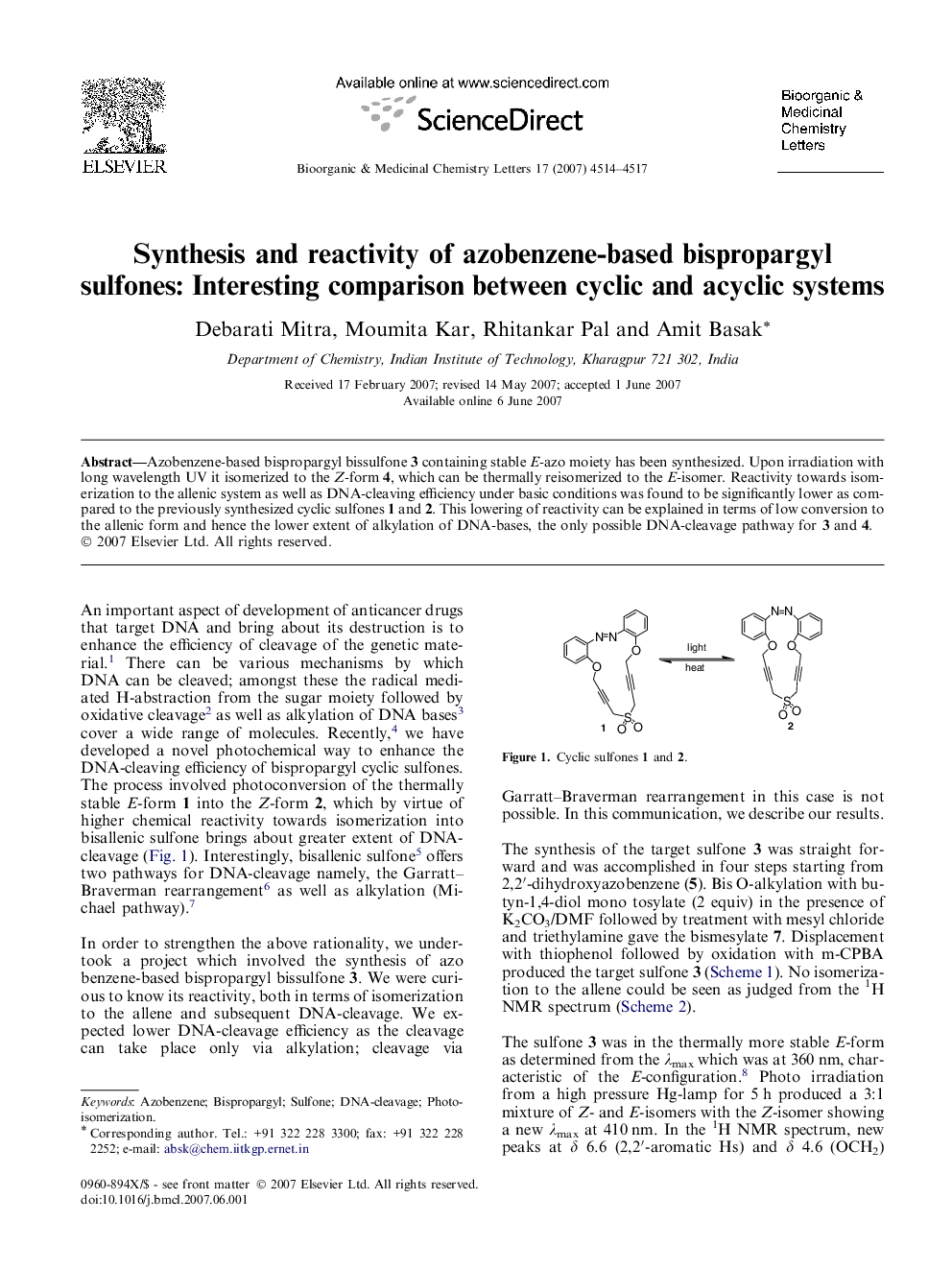
Azobenzene-based bispropargyl bissulfone 3 containing stable E-azo moiety has been synthesized. Upon irradiation with long wavelength UV it isomerized to the Z-form 4, which can be thermally reisomerized to the E-isomer. Reactivity towards isomerization to the allenic system as well as DNA-cleaving efficiency under basic conditions was found to be significantly lower as compared to the previously synthesized cyclic sulfones 1 and 2. This lowering of reactivity can be explained in terms of low conversion to the allenic form and hence the lower extent of alkylation of DNA-bases, the only possible DNA-cleavage pathway for 3 and 4.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Debarati Mitra, Moumita Kar, Rhitankar Pal, Amit Basak,