| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1377460 | Bioorganic & Medicinal Chemistry Letters | 2006 | 6 Pages |
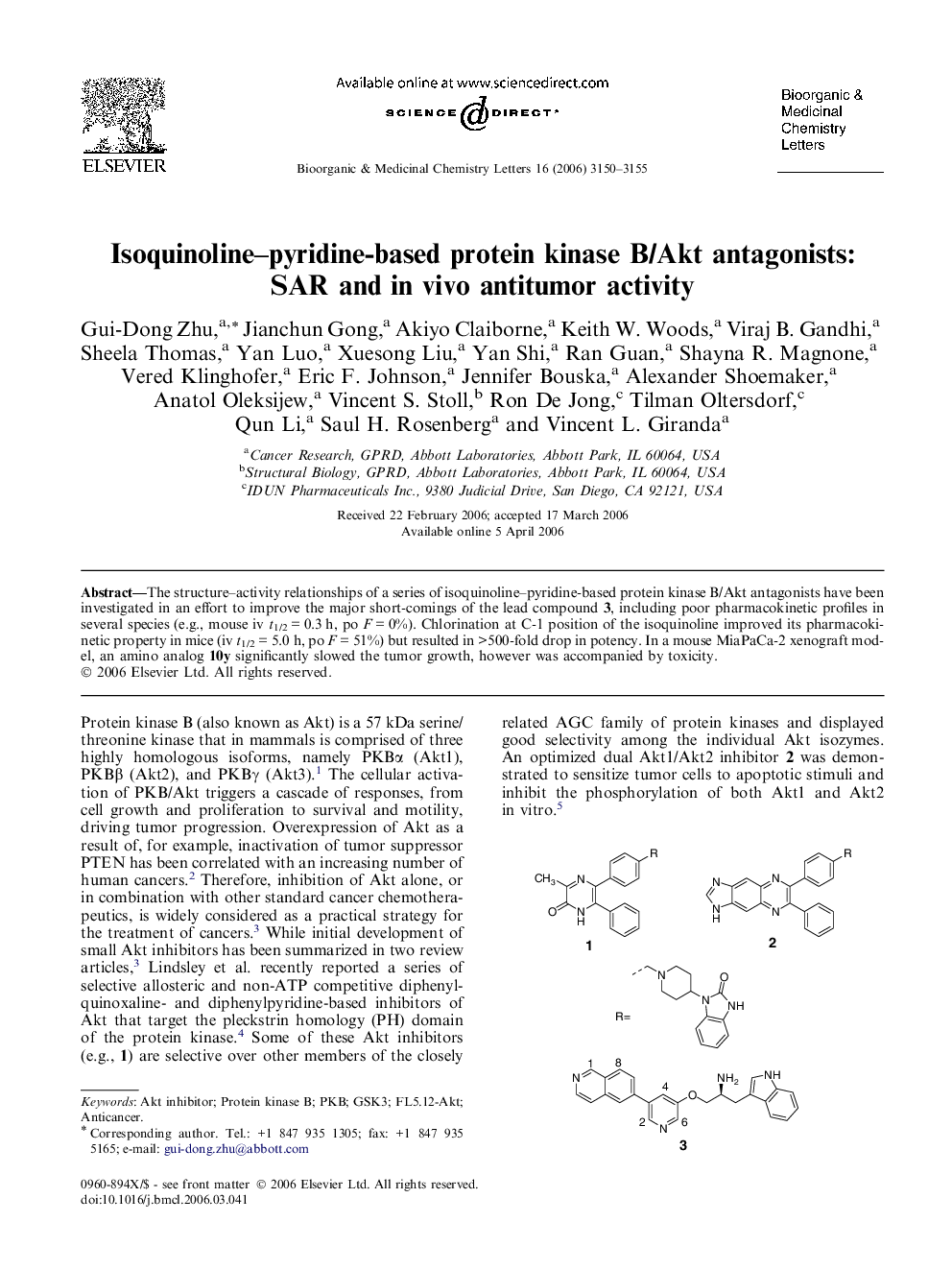
The structure–activity relationships of a series of isoquinoline–pyridine-based protein kinase B/Akt antagonists have been investigated in an effort to improve the major short-comings of the lead compound 3, including poor pharmacokinetic profiles in several species (e.g., mouse iv t1/2 = 0.3 h, po F = 0%). Chlorination at C-1 position of the isoquinoline improved its pharmacokinetic property in mice (iv t1/2 = 5.0 h, po F = 51%) but resulted in >500-fold drop in potency. In a mouse MiaPaCa-2 xenograft model, an amino analog 10y significantly slowed the tumor growth, however was accompanied by toxicity.
Graphical abstractA series of 27 isoquinoline–pyridine-based derivatives were synthesized and evaluated as protein kinase B/Akt antagonists. An amino analog (R5 = NH2) demonstrated good efficacy in a mouse MiaPaCa-2 xenograft model, but with accompanying toxicity.Figure optionsDownload full-size imageDownload as PowerPoint slide