| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1377700 | Bioorganic & Medicinal Chemistry Letters | 2006 | 4 Pages |
Abstract
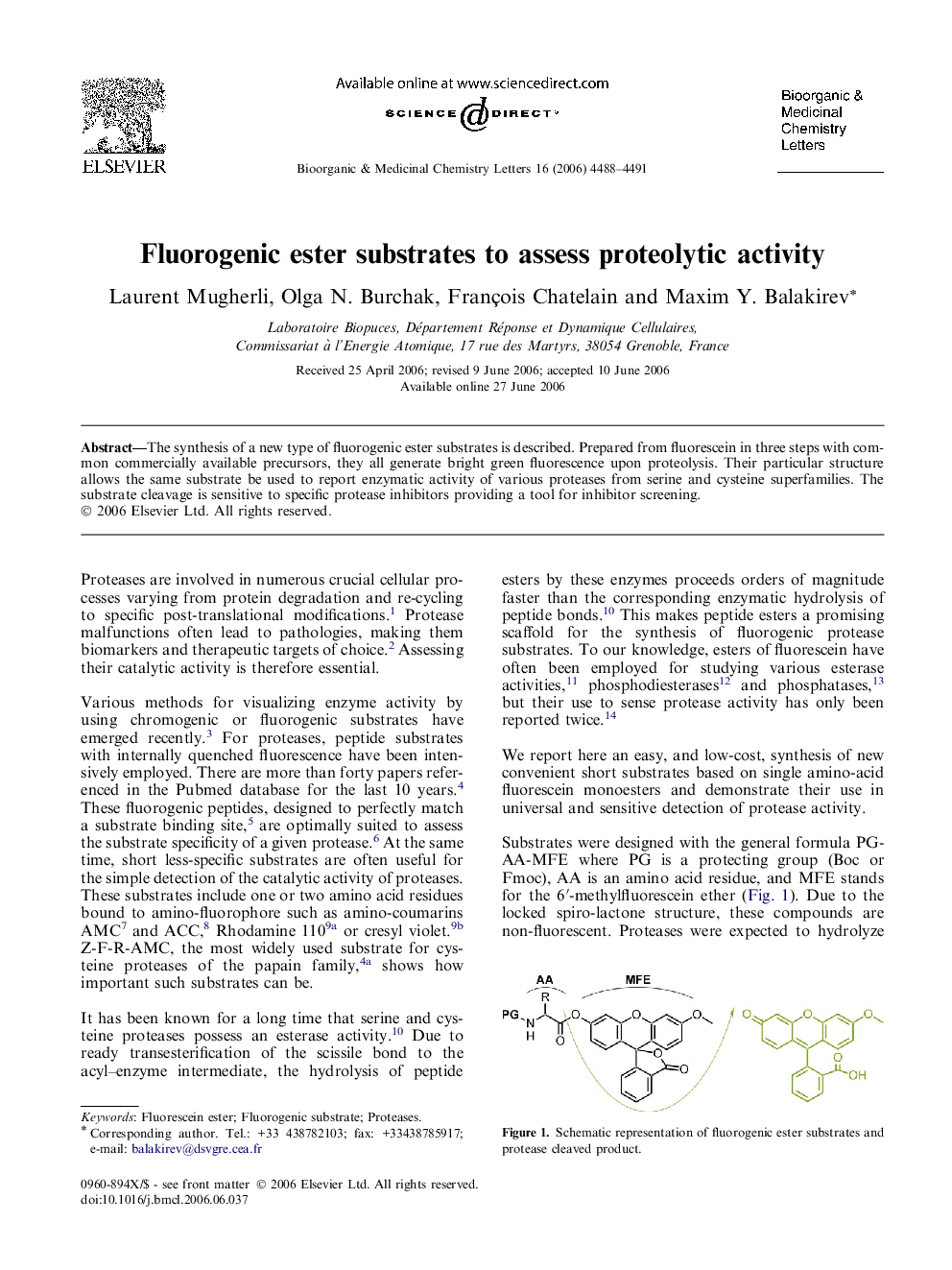
The synthesis of a new type of fluorogenic ester substrates is described. Prepared from fluorescein in three steps with common commercially available precursors, they all generate bright green fluorescence upon proteolysis. Their particular structure allows the same substrate be used to report enzymatic activity of various proteases from serine and cysteine superfamilies. The substrate cleavage is sensitive to specific protease inhibitors providing a tool for inhibitor screening.
Graphical abstractThe synthesis of a new type of fluorogenic ester substrates is described. Prepared from fluorescein in three steps with common commercially available precursors, they generate bright green fluorescence upon proteolysis.
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Laurent Mugherli, Olga N. Burchak, François Chatelain, Maxim Y. Balakirev,