| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1378246 | Bioorganic & Medicinal Chemistry Letters | 2007 | 5 Pages |
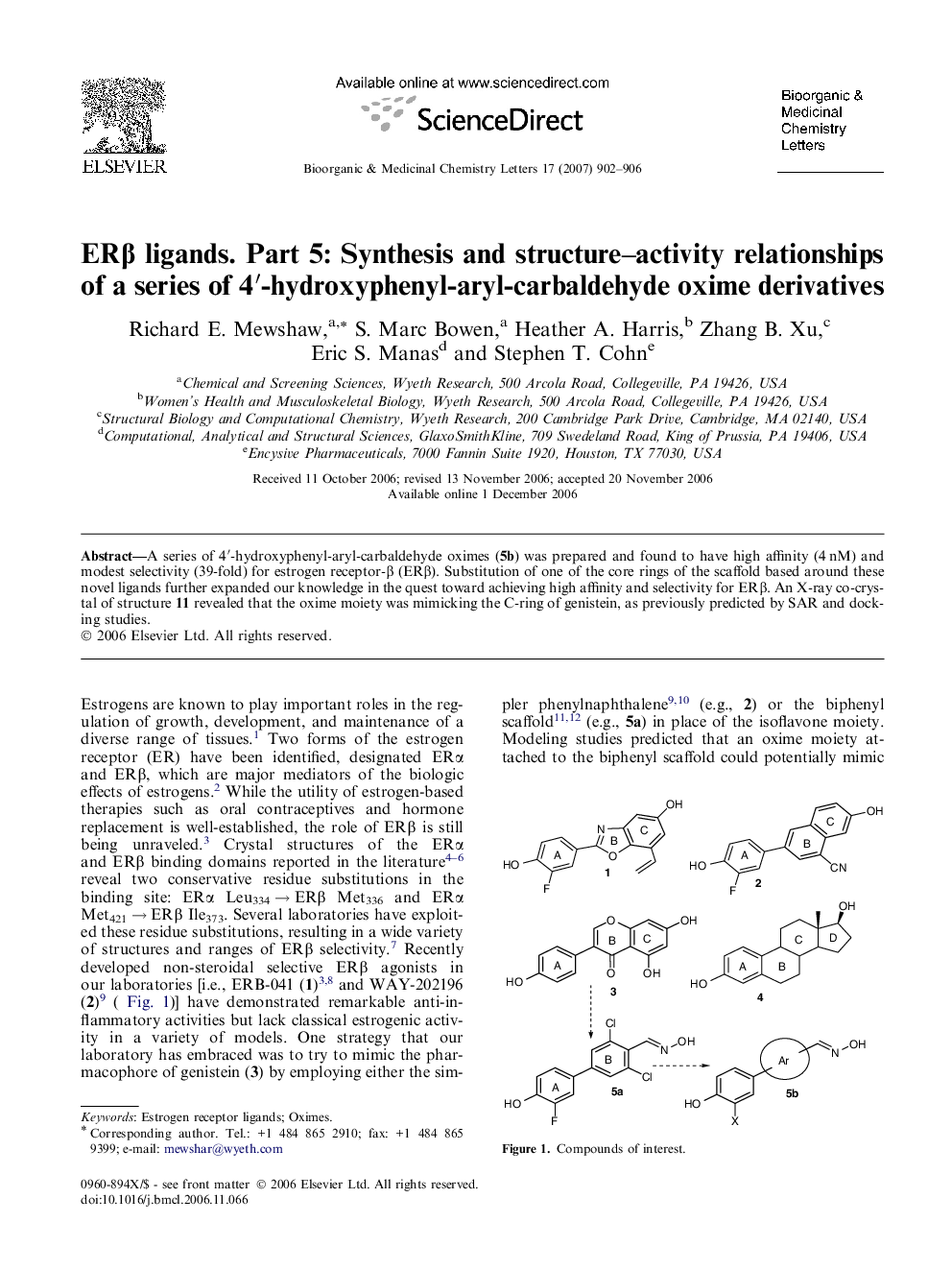
A series of 4′-hydroxyphenyl-aryl-carbaldehyde oximes (5b) was prepared and found to have high affinity (4 nM) and modest selectivity (39-fold) for estrogen receptor-β (ERβ). Substitution of one of the core rings of the scaffold based around these novel ligands further expanded our knowledge in the quest toward achieving high affinity and selectivity for ERβ. An X-ray co-crystal of structure 11 revealed that the oxime moiety was mimicking the C-ring of genistein, as previously predicted by SAR and docking studies.
Graphical abstractA series of 4′-hydroxyphenyl-aryl-carbaldehyde oximes (5b) was prepared and found to have high-affinity (4 nM) and modest selectivity (39-fold) for estrogen receptor-β.Figure optionsDownload full-size imageDownload as PowerPoint slide