| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1378247 | Bioorganic & Medicinal Chemistry Letters | 2007 | 4 Pages |
Abstract
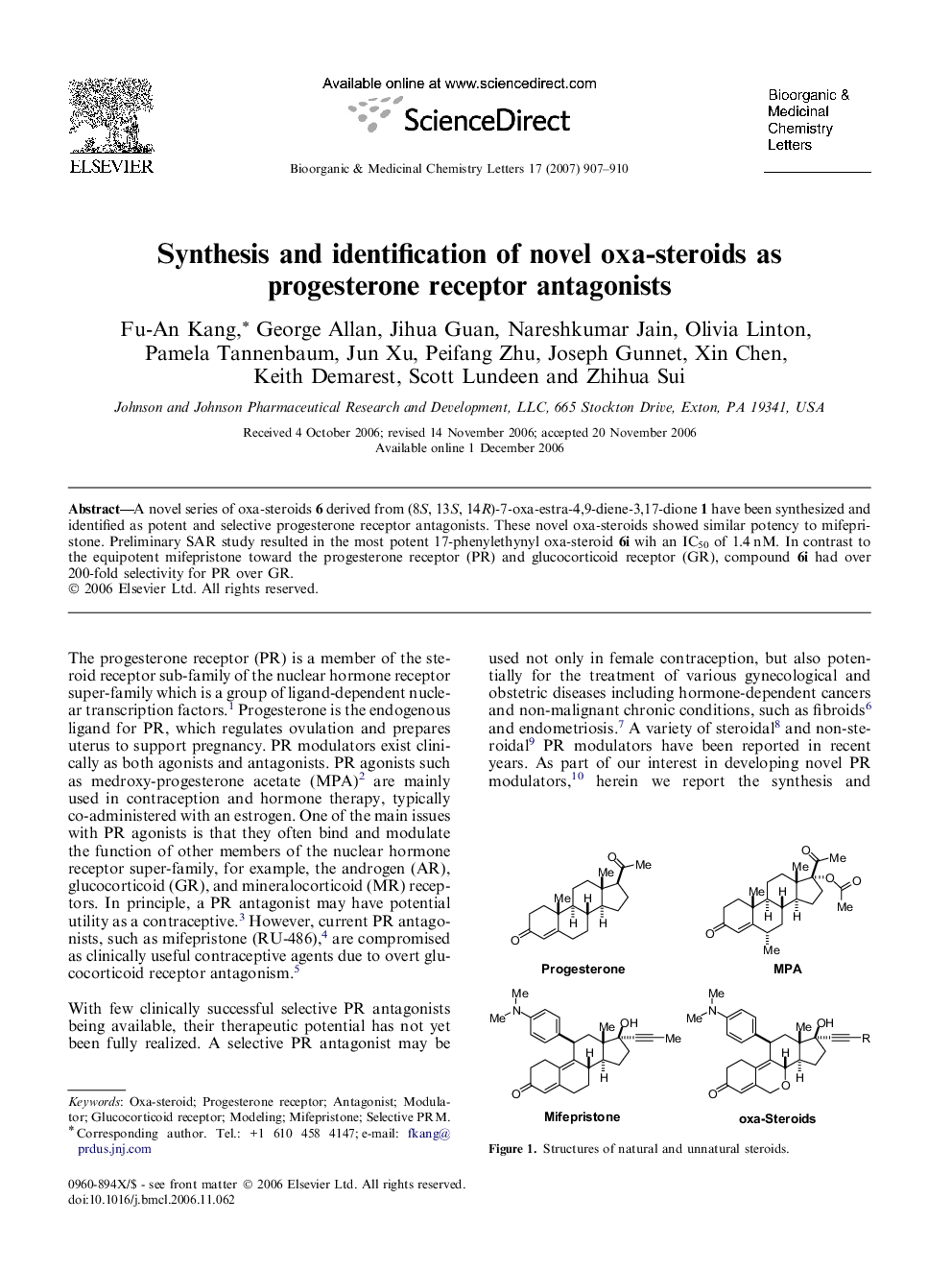
A novel series of oxa-steroids 6 derived from (8S, 13S, 14R)-7-oxa-estra-4,9-diene-3,17-dione 1 have been synthesized and identified as potent and selective progesterone receptor antagonists. These novel oxa-steroids showed similar potency to mifepristone. Preliminary SAR study resulted in the most potent 17-phenylethynyl oxa-steroid 6i wih an IC50 of 1.4 nM. In contrast to the equipotent mifepristone toward the progesterone receptor (PR) and glucocorticoid receptor (GR), compound 6i had over 200-fold selectivity for PR over GR.
Graphical abstractNovel 7-oxa-steroids were synthesized and indentified as new potent and selective progesterone receptor antagonists.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Fu-An Kang, George Allan, Jihua Guan, Nareshkumar Jain, Olivia Linton, Pamela Tannenbaum, Jun Xu, Peifang Zhu, Joseph Gunnet, Xin Chen, Keith Demarest, Scott Lundeen, Zhihua Sui,