| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1379274 | Bioorganic & Medicinal Chemistry Letters | 2006 | 4 Pages |
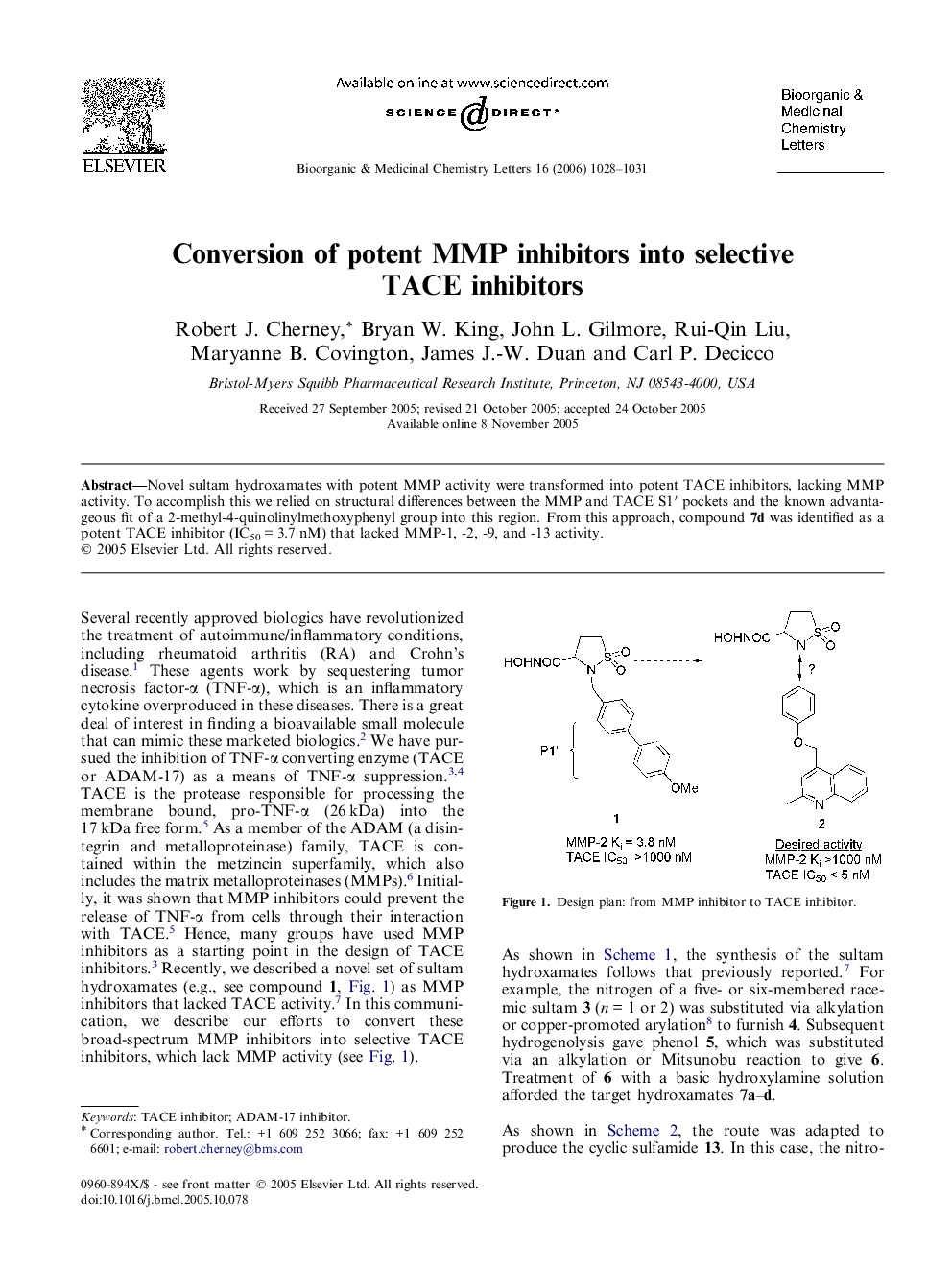
Novel sultam hydroxamates with potent MMP activity were transformed into potent TACE inhibitors, lacking MMP activity. To accomplish this we relied on structural differences between the MMP and TACE S1′ pockets and the known advantageous fit of a 2-methyl-4-quinolinylmethoxyphenyl group into this region. From this approach, compound 7d was identified as a potent TACE inhibitor (IC50 = 3.7 nM) that lacked MMP-1, -2, -9, and -13 activity.
Graphical abstractNovel sultam hydroxamates with potent MMP activity were transformed into potent TACE inhibitors, lacking MMP activity. From this effort, compound 7d was identified as a potent TACE inhibitor (IC50 = 3.7 nM) that lacked MMP-1, -2, -9, and -13 activity.Figure optionsDownload full-size imageDownload as PowerPoint slide