| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1379369 | Bioorganic & Medicinal Chemistry Letters | 2005 | 5 Pages |
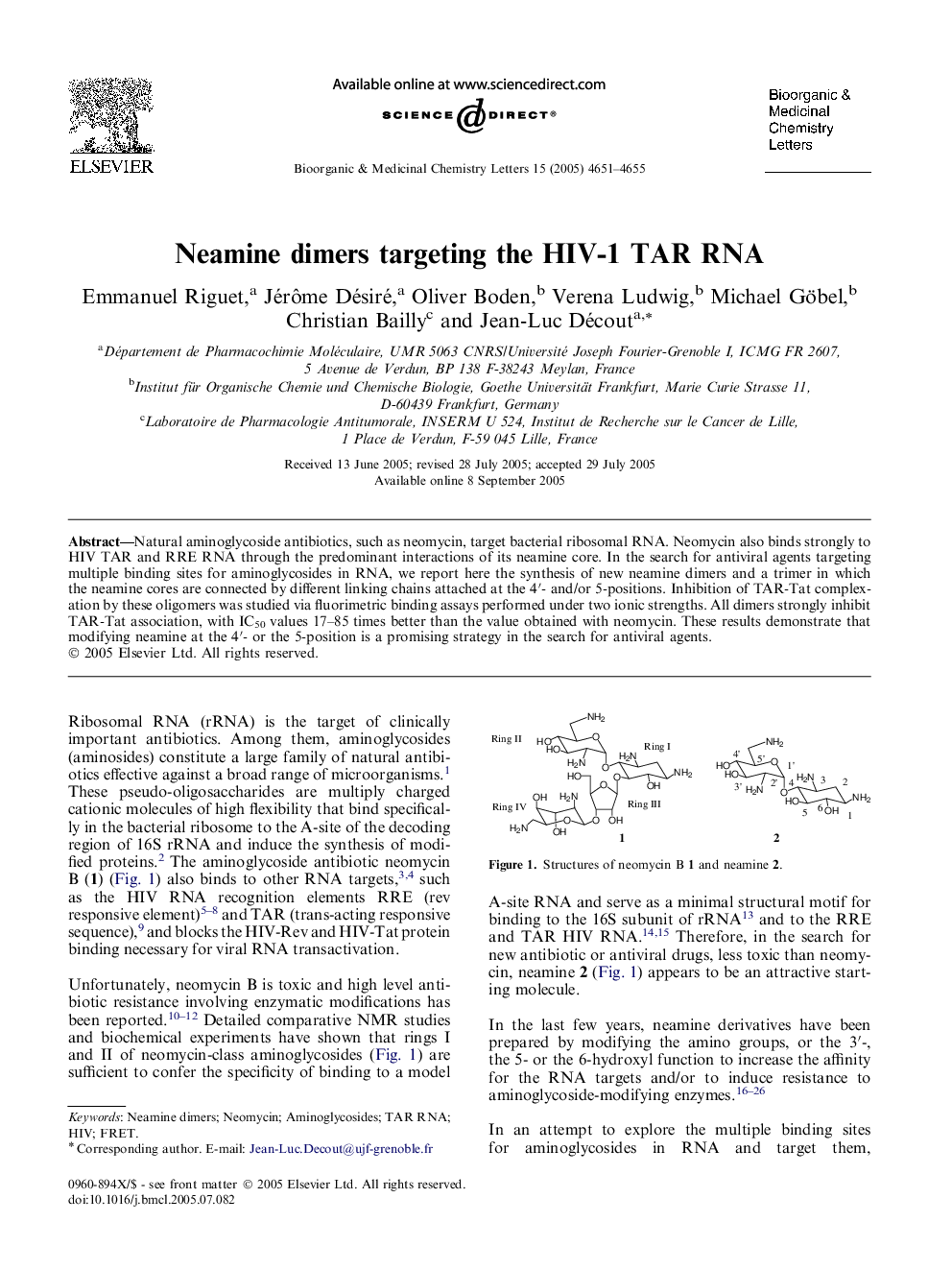
Natural aminoglycoside antibiotics, such as neomycin, target bacterial ribosomal RNA. Neomycin also binds strongly to HIV TAR and RRE RNA through the predominant interactions of its neamine core. In the search for antiviral agents targeting multiple binding sites for aminoglycosides in RNA, we report here the synthesis of new neamine dimers and a trimer in which the neamine cores are connected by different linking chains attached at the 4′- and/or 5-positions. Inhibition of TAR-Tat complexation by these oligomers was studied via fluorimetric binding assays performed under two ionic strengths. All dimers strongly inhibit TAR-Tat association, with IC50 values 17–85 times better than the value obtained with neomycin. These results demonstrate that modifying neamine at the 4′- or the 5-position is a promising strategy in the search for antiviral agents.
Graphical abstractThe natural antibiotic agent neomycin binds strongly to HIV TAR RNA through the predominant interactions of its neamine core and inhibits the TAR-Tat protein association necessary for viral RNA transactivation. In the search for new antiviral agents, neamine dimers were found to be able to inhibit the TAR-Tat association, with IC50 values 17–85 times better than that obtained with neomycin.Figure optionsDownload full-size imageDownload as PowerPoint slide