| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145256 | Chemical Engineering Journal | 2016 | 6 Pages |
•Synergistic treatment of different waste streams in coal-fired power plant.•Self-sustaining carbon sequestration by electrolytic carbonation of coal fly ash.•High purity CaCO3 production and promotion of fly ash as renewable material.•Low energy, cost and environmental affect for carbon mitigation.
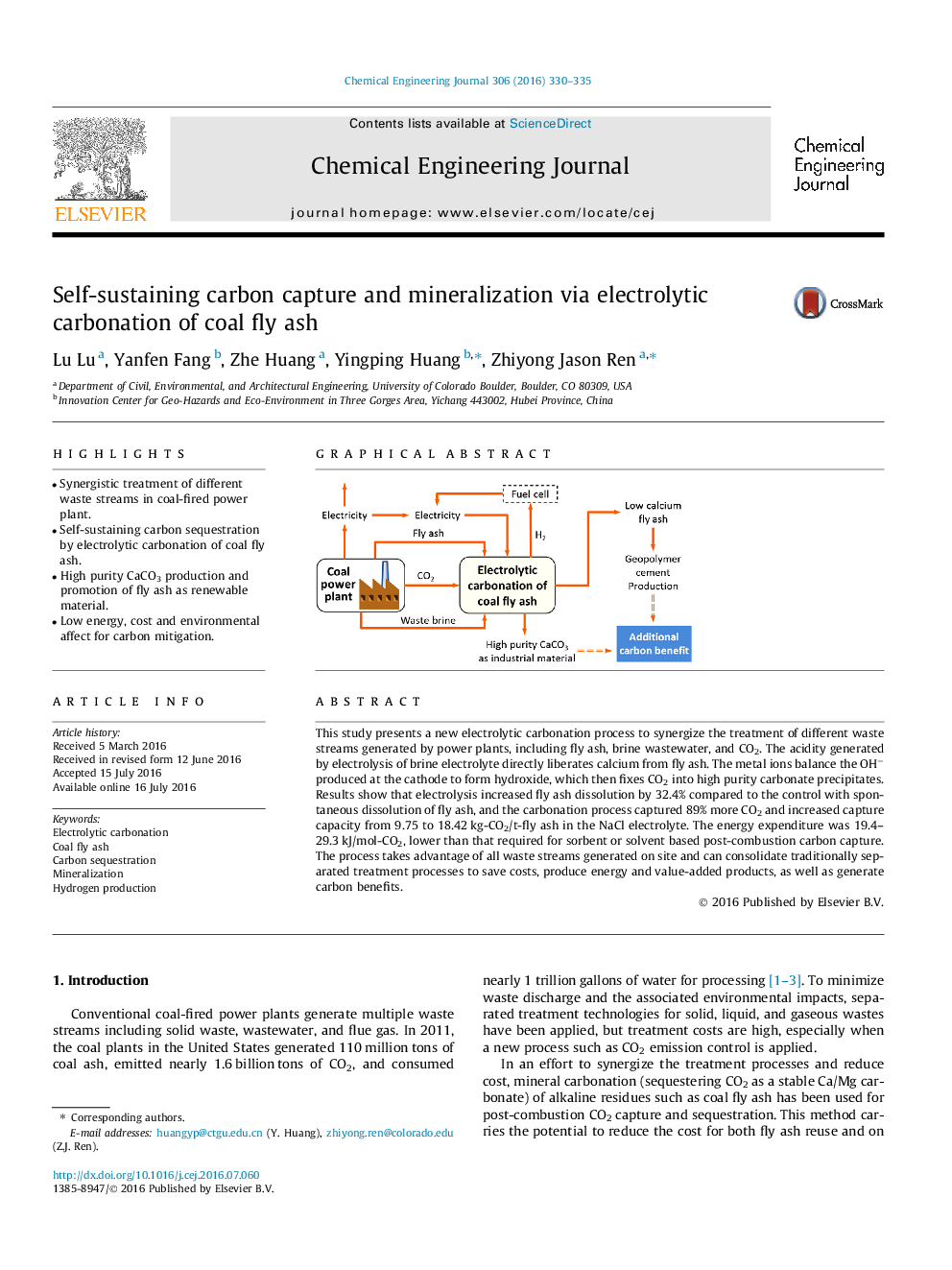
This study presents a new electrolytic carbonation process to synergize the treatment of different waste streams generated by power plants, including fly ash, brine wastewater, and CO2. The acidity generated by electrolysis of brine electrolyte directly liberates calcium from fly ash. The metal ions balance the OH− produced at the cathode to form hydroxide, which then fixes CO2 into high purity carbonate precipitates. Results show that electrolysis increased fly ash dissolution by 32.4% compared to the control with spontaneous dissolution of fly ash, and the carbonation process captured 89% more CO2 and increased capture capacity from 9.75 to 18.42 kg-CO2/t-fly ash in the NaCl electrolyte. The energy expenditure was 19.4–29.3 kJ/mol-CO2, lower than that required for sorbent or solvent based post-combustion carbon capture. The process takes advantage of all waste streams generated on site and can consolidate traditionally separated treatment processes to save costs, produce energy and value-added products, as well as generate carbon benefits.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide