| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145267 | Chemical Engineering Journal | 2016 | 10 Pages |
•The presence of HSO5− with gamma-ray promoted removal efficiency of chlorendic acid.•The activation of HSO5− by gamma-ray and catalyst yield OH and SO4−.•The radical scavengers inhibited the efficiency of OH and SO4−.•Second-order rate constants of chlorendic acid with eaq−, OH, and SO4− were determined.•Degradation pathways were proposed from the nature of identified by-products.
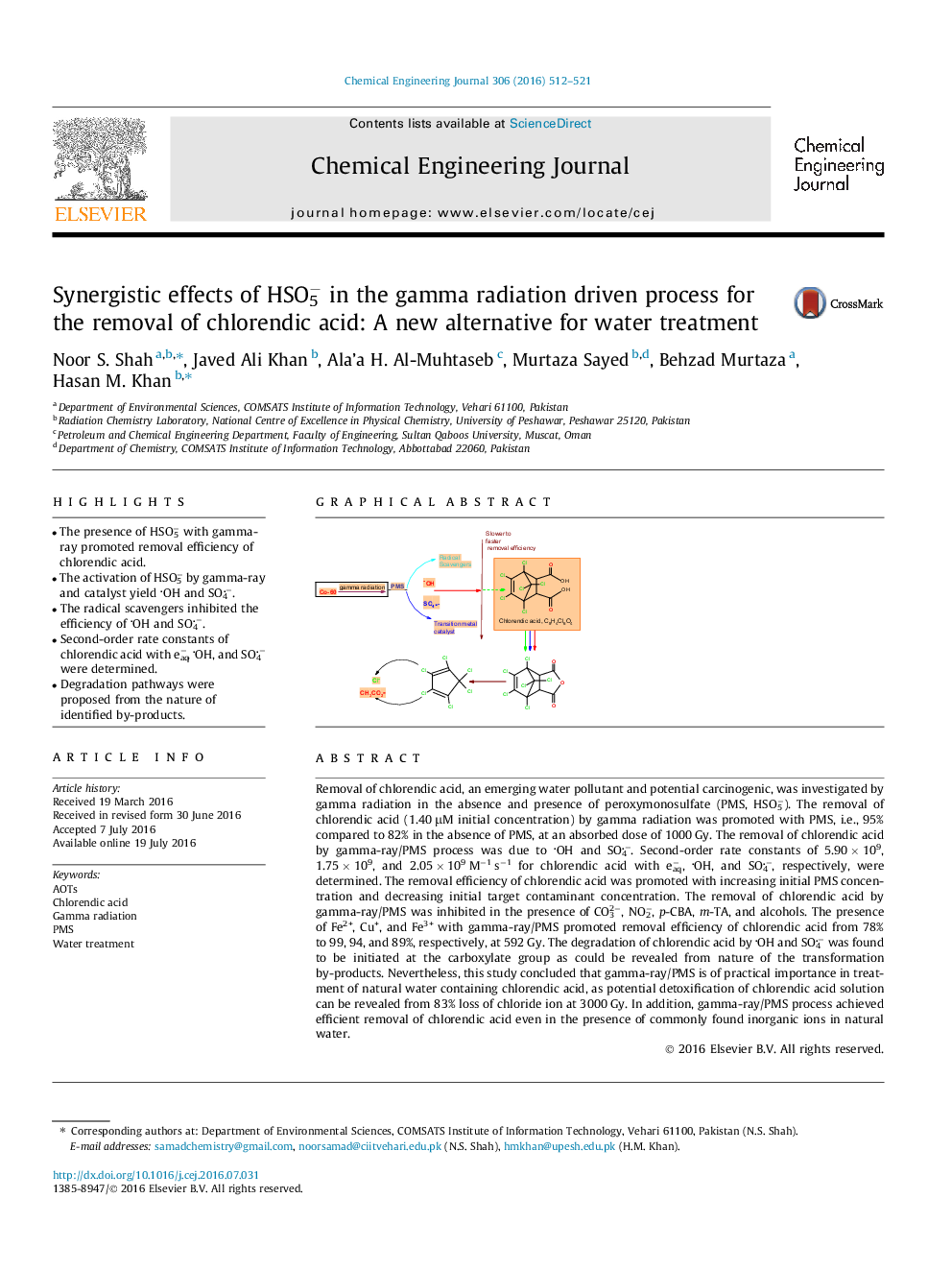
Removal of chlorendic acid, an emerging water pollutant and potential carcinogenic, was investigated by gamma radiation in the absence and presence of peroxymonosulfate (PMS, HSO5−). The removal of chlorendic acid (1.40 μM initial concentration) by gamma radiation was promoted with PMS, i.e., 95% compared to 82% in the absence of PMS, at an absorbed dose of 1000 Gy. The removal of chlorendic acid by gamma-ray/PMS process was due to OH and SO4−. Second-order rate constants of 5.90 × 109, 1.75 × 109, and 2.05 × 109 M−1 s−1 for chlorendic acid with eaq−, OH, and SO4−, respectively, were determined. The removal efficiency of chlorendic acid was promoted with increasing initial PMS concentration and decreasing initial target contaminant concentration. The removal of chlorendic acid by gamma-ray/PMS was inhibited in the presence of CO32−, NO2−, p-CBA, m-TA, and alcohols. The presence of Fe2+, Cu+, and Fe3+ with gamma-ray/PMS promoted removal efficiency of chlorendic acid from 78% to 99, 94, and 89%, respectively, at 592 Gy. The degradation of chlorendic acid by OH and SO4− was found to be initiated at the carboxylate group as could be revealed from nature of the transformation by-products. Nevertheless, this study concluded that gamma-ray/PMS is of practical importance in treatment of natural water containing chlorendic acid, as potential detoxification of chlorendic acid solution can be revealed from 83% loss of chloride ion at 3000 Gy. In addition, gamma-ray/PMS process achieved efficient removal of chlorendic acid even in the presence of commonly found inorganic ions in natural water.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide