| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145359 | Chemical Engineering Journal | 2016 | 10 Pages |
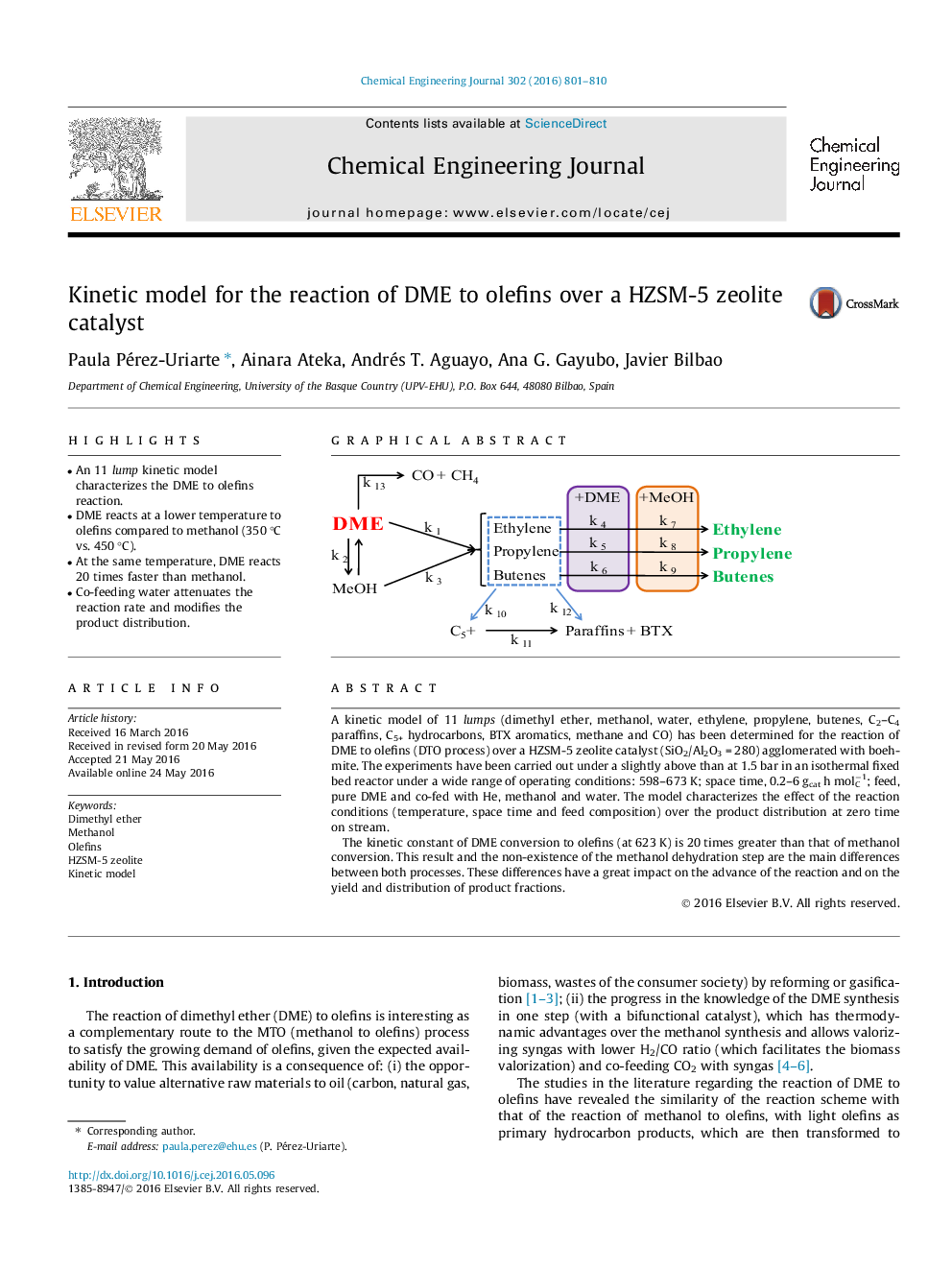
•An 11 lump kinetic model characterizes the DME to olefins reaction.•DME reacts at a lower temperature to olefins compared to methanol (350 °C vs. 450 °C).•At the same temperature, DME reacts 20 times faster than methanol.•Co-feeding water attenuates the reaction rate and modifies the product distribution.
A kinetic model of 11 lumps (dimethyl ether, methanol, water, ethylene, propylene, butenes, C2–C4 paraffins, C5+ hydrocarbons, BTX aromatics, methane and CO) has been determined for the reaction of DME to olefins (DTO process) over a HZSM-5 zeolite catalyst (SiO2/Al2O3 = 280) agglomerated with boehmite. The experiments have been carried out under a slightly above than at 1.5 bar in an isothermal fixed bed reactor under a wide range of operating conditions: 598–673 K; space time, 0.2–6 gcat h molC−1; feed, pure DME and co-fed with He, methanol and water. The model characterizes the effect of the reaction conditions (temperature, space time and feed composition) over the product distribution at zero time on stream.The kinetic constant of DME conversion to olefins (at 623 K) is 20 times greater than that of methanol conversion. This result and the non-existence of the methanol dehydration step are the main differences between both processes. These differences have a great impact on the advance of the reaction and on the yield and distribution of product fractions.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide