| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145426 | Chemical Engineering Journal | 2016 | 8 Pages |
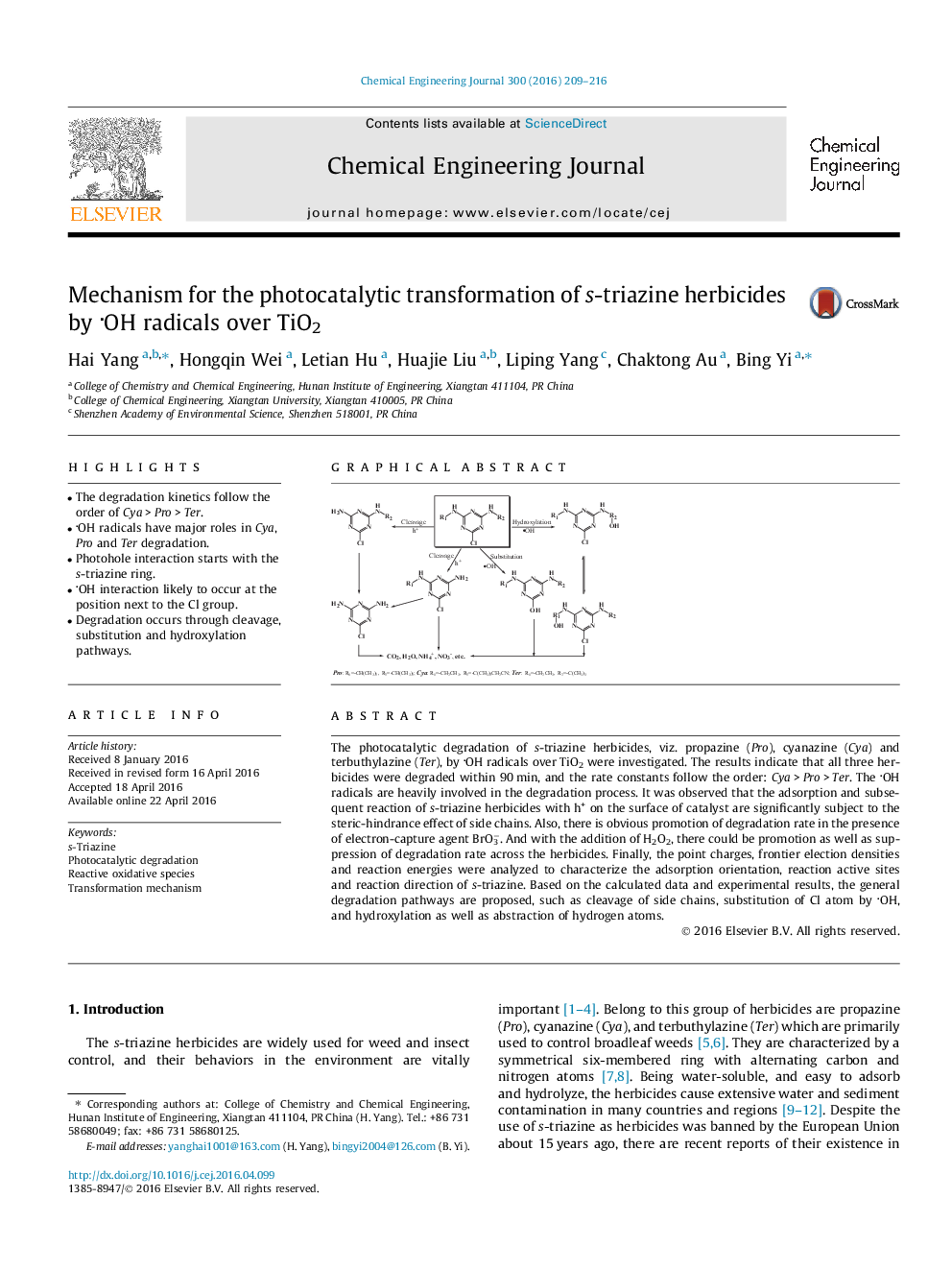
•The degradation kinetics follow the order of Cya > Pro > Ter.•OH radicals have major roles in Cya, Pro and Ter degradation.•Photohole interaction starts with the s-triazine ring.•OH interaction likely to occur at the position next to the Cl group.•Degradation occurs through cleavage, substitution and hydroxylation pathways.
The photocatalytic degradation of s-triazine herbicides, viz. propazine (Pro), cyanazine (Cya) and terbuthylazine (Ter), by OH radicals over TiO2 were investigated. The results indicate that all three herbicides were degraded within 90 min, and the rate constants follow the order: Cya > Pro > Ter. The OH radicals are heavily involved in the degradation process. It was observed that the adsorption and subsequent reaction of s-triazine herbicides with h+ on the surface of catalyst are significantly subject to the steric-hindrance effect of side chains. Also, there is obvious promotion of degradation rate in the presence of electron-capture agent BrO3−. And with the addition of H2O2, there could be promotion as well as suppression of degradation rate across the herbicides. Finally, the point charges, frontier election densities and reaction energies were analyzed to characterize the adsorption orientation, reaction active sites and reaction direction of s-triazine. Based on the calculated data and experimental results, the general degradation pathways are proposed, such as cleavage of side chains, substitution of Cl atom by OH, and hydroxylation as well as abstraction of hydrogen atoms.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide