| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145461 | Chemical Engineering Journal | 2016 | 13 Pages |
•A novel approach for the efficient removal of MB was proposed.•SDS solubilization and Ca2+ effect were combined to reduce the solubility of MB.•MB precipitated from aqueous solution by adsorbing onto the Ca(DS)2 particles.•The Ca(DS)2 particles adsorbed MB were flocculated by MBFGA1.
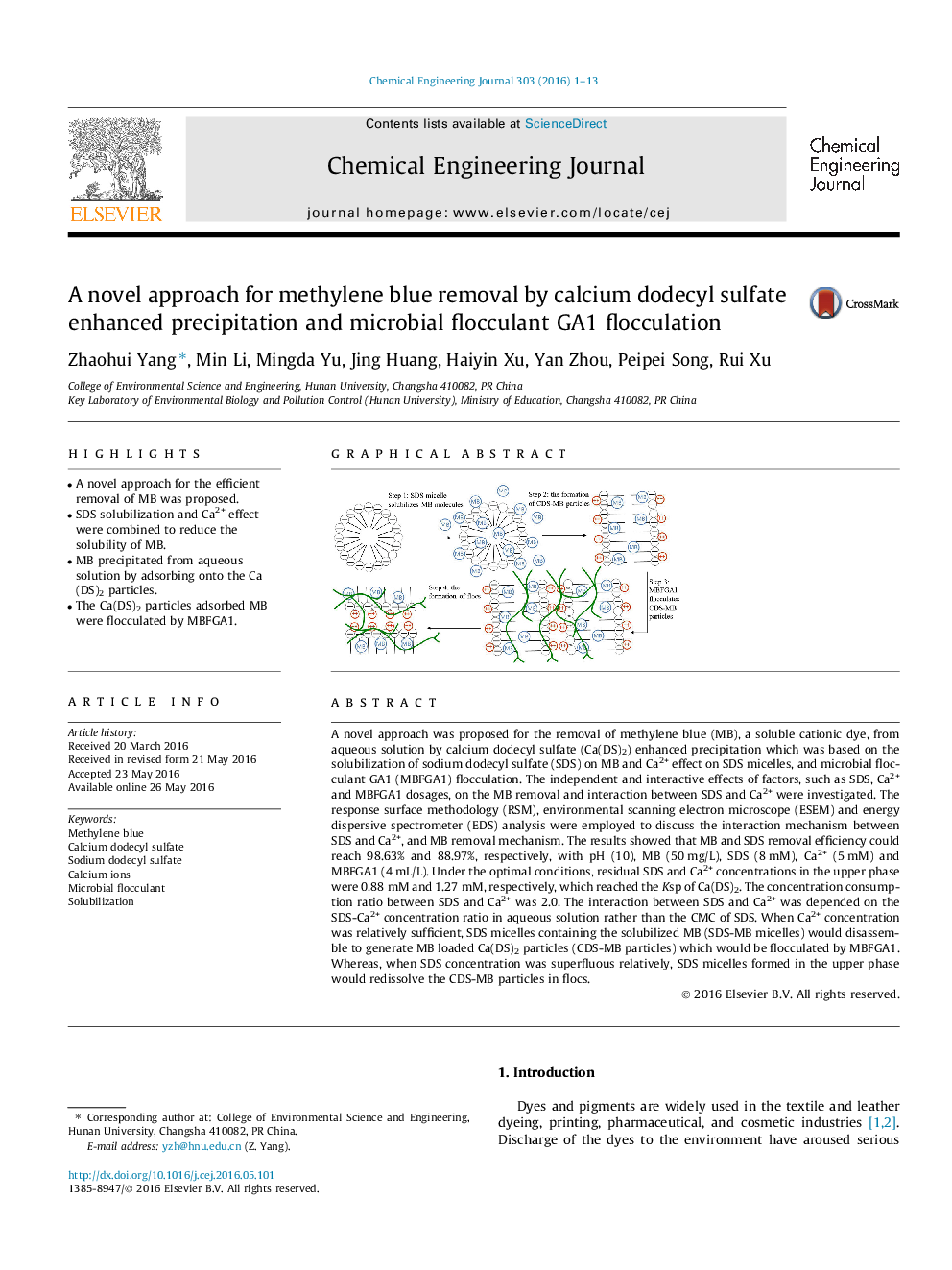
A novel approach was proposed for the removal of methylene blue (MB), a soluble cationic dye, from aqueous solution by calcium dodecyl sulfate (Ca(DS)2) enhanced precipitation which was based on the solubilization of sodium dodecyl sulfate (SDS) on MB and Ca2+ effect on SDS micelles, and microbial flocculant GA1 (MBFGA1) flocculation. The independent and interactive effects of factors, such as SDS, Ca2+ and MBFGA1 dosages, on the MB removal and interaction between SDS and Ca2+ were investigated. The response surface methodology (RSM), environmental scanning electron microscope (ESEM) and energy dispersive spectrometer (EDS) analysis were employed to discuss the interaction mechanism between SDS and Ca2+, and MB removal mechanism. The results showed that MB and SDS removal efficiency could reach 98.63% and 88.97%, respectively, with pH (10), MB (50 mg/L), SDS (8 mM), Ca2+ (5 mM) and MBFGA1 (4 mL/L). Under the optimal conditions, residual SDS and Ca2+ concentrations in the upper phase were 0.88 mM and 1.27 mM, respectively, which reached the Ksp of Ca(DS)2. The concentration consumption ratio between SDS and Ca2+ was 2.0. The interaction between SDS and Ca2+ was depended on the SDS-Ca2+ concentration ratio in aqueous solution rather than the CMC of SDS. When Ca2+ concentration was relatively sufficient, SDS micelles containing the solubilized MB (SDS-MB micelles) would disassemble to generate MB loaded Ca(DS)2 particles (CDS-MB particles) which would be flocculated by MBFGA1. Whereas, when SDS concentration was superfluous relatively, SDS micelles formed in the upper phase would redissolve the CDS-MB particles in flocs.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide