| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145464 | Chemical Engineering Journal | 2016 | 9 Pages |
•Hydrogen titanate nanotube shows high H2 uptake of 12.5 mmol/g at 25 °C and 1 atm.•Amine-modified hydrogen titanate shows quite good CO2 uptake of 1.2 mmol/g at 25 °C and 1 atm.•Hydrogen titanate nanotube shows good reversibility for H2 and CO2 gas uptake.•Selectivity for gas uptake was observed under a gas flow mixed with N2 gas.
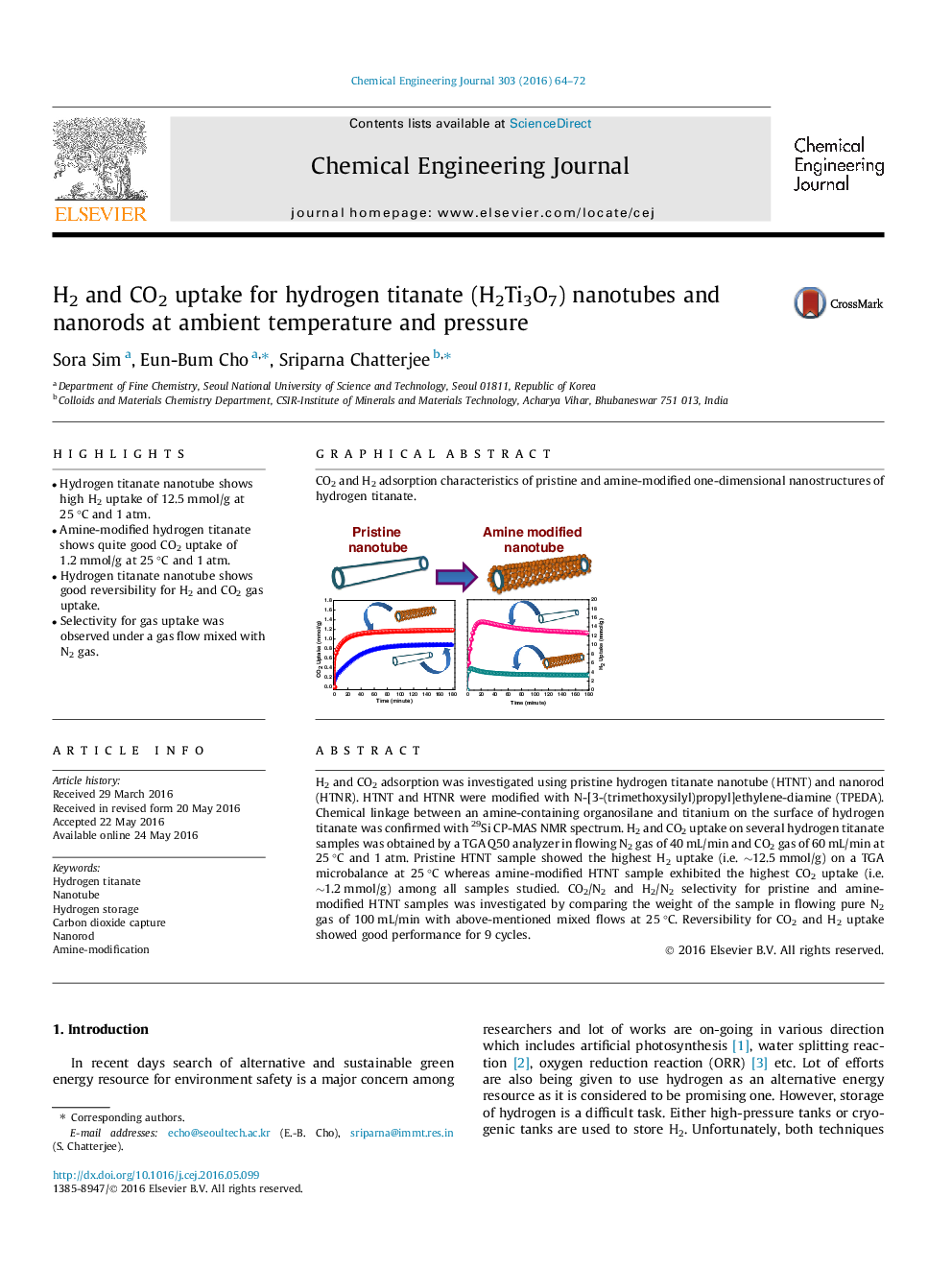
H2 and CO2 adsorption was investigated using pristine hydrogen titanate nanotube (HTNT) and nanorod (HTNR). HTNT and HTNR were modified with N-[3-(trimethoxysilyl)propyl]ethylene-diamine (TPEDA). Chemical linkage between an amine-containing organosilane and titanium on the surface of hydrogen titanate was confirmed with 29Si CP-MAS NMR spectrum. H2 and CO2 uptake on several hydrogen titanate samples was obtained by a TGA Q50 analyzer in flowing N2 gas of 40 mL/min and CO2 gas of 60 mL/min at 25 °C and 1 atm. Pristine HTNT sample showed the highest H2 uptake (i.e. ∼12.5 mmol/g) on a TGA microbalance at 25 °C whereas amine-modified HTNT sample exhibited the highest CO2 uptake (i.e. ∼1.2 mmol/g) among all samples studied. CO2/N2 and H2/N2 selectivity for pristine and amine-modified HTNT samples was investigated by comparing the weight of the sample in flowing pure N2 gas of 100 mL/min with above-mentioned mixed flows at 25 °C. Reversibility for CO2 and H2 uptake showed good performance for 9 cycles.
Graphical abstractCO2 and H2 adsorption characteristics of pristine and amine-modified one-dimensional nanostructures of hydrogen titanate.Figure optionsDownload full-size imageDownload as PowerPoint slide