| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145485 | Chemical Engineering Journal | 2016 | 9 Pages |
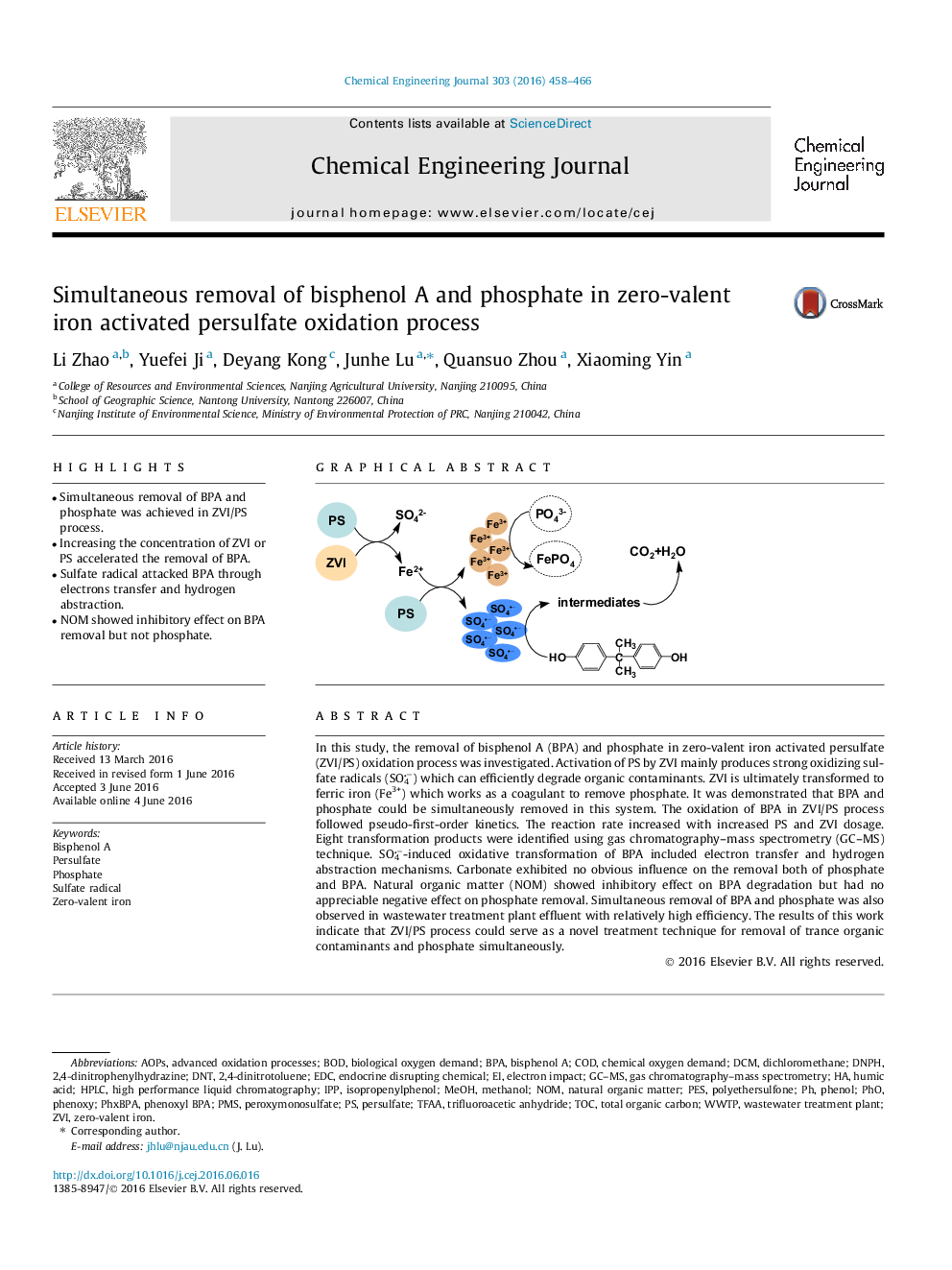
•Simultaneous removal of BPA and phosphate was achieved in ZVI/PS process.•Increasing the concentration of ZVI or PS accelerated the removal of BPA.•Sulfate radical attacked BPA through electrons transfer and hydrogen abstraction.•NOM showed inhibitory effect on BPA removal but not phosphate.
In this study, the removal of bisphenol A (BPA) and phosphate in zero-valent iron activated persulfate (ZVI/PS) oxidation process was investigated. Activation of PS by ZVI mainly produces strong oxidizing sulfate radicals (SO4−) which can efficiently degrade organic contaminants. ZVI is ultimately transformed to ferric iron (Fe3+) which works as a coagulant to remove phosphate. It was demonstrated that BPA and phosphate could be simultaneously removed in this system. The oxidation of BPA in ZVI/PS process followed pseudo-first-order kinetics. The reaction rate increased with increased PS and ZVI dosage. Eight transformation products were identified using gas chromatography–mass spectrometry (GC–MS) technique. SO4−-induced oxidative transformation of BPA included electron transfer and hydrogen abstraction mechanisms. Carbonate exhibited no obvious influence on the removal both of phosphate and BPA. Natural organic matter (NOM) showed inhibitory effect on BPA degradation but had no appreciable negative effect on phosphate removal. Simultaneous removal of BPA and phosphate was also observed in wastewater treatment plant effluent with relatively high efficiency. The results of this work indicate that ZVI/PS process could serve as a novel treatment technique for removal of trance organic contaminants and phosphate simultaneously.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide