| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145760 | Chemical Engineering Journal | 2016 | 11 Pages |
•Ordered mesoporous carbons are oxidized by ammonium persulfate at 60 °C.•Longer oxidation time is favorable for generation of high density of COOH groups.•Dispersion stability of mesoporous carbon is analyzed by optical methods.•Higher transmission intensity is related with more serious sedimentation.•The dispersion of functionalized carbons are much more stable than pure materials.
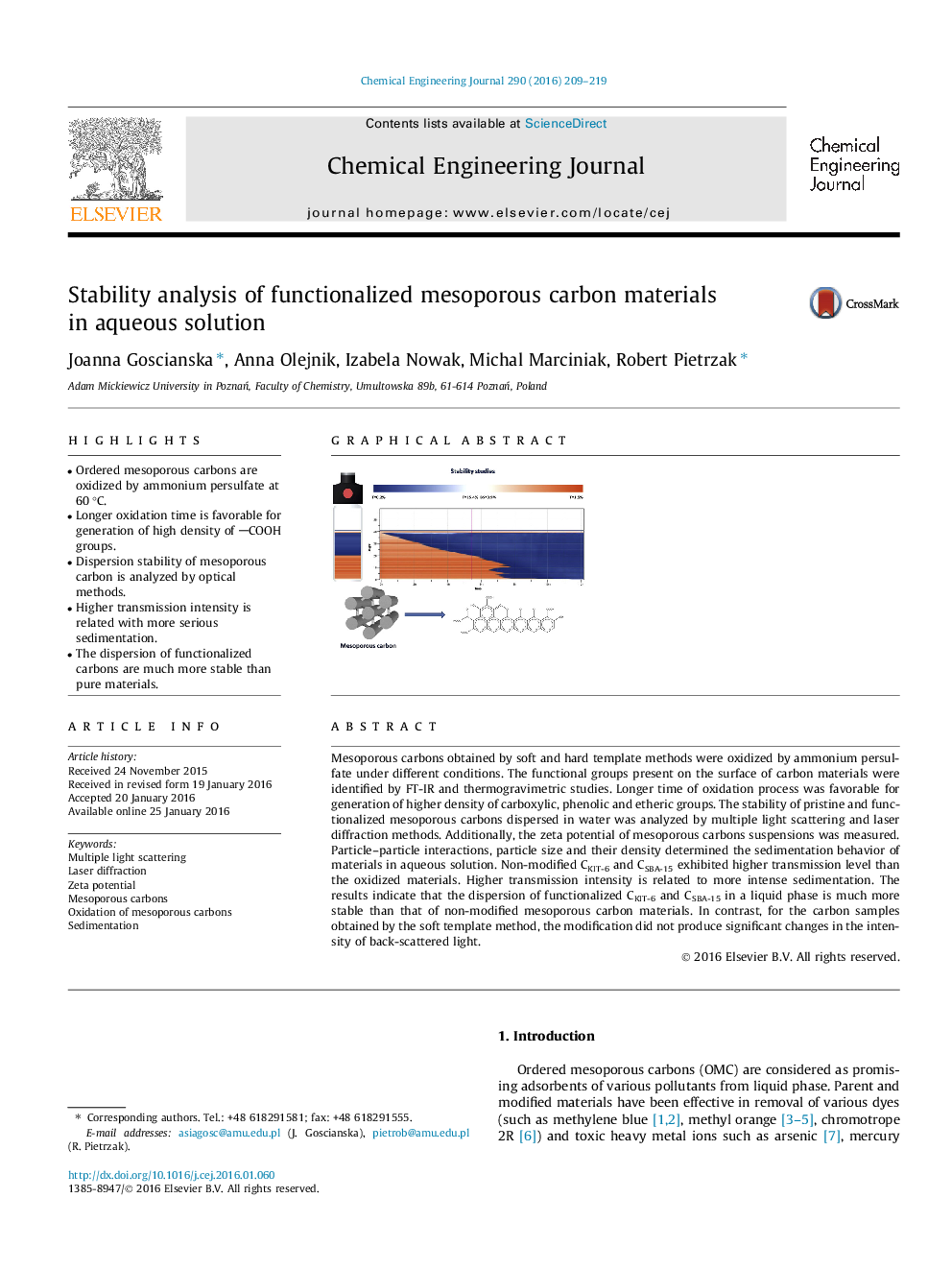
Mesoporous carbons obtained by soft and hard template methods were oxidized by ammonium persulfate under different conditions. The functional groups present on the surface of carbon materials were identified by FT-IR and thermogravimetric studies. Longer time of oxidation process was favorable for generation of higher density of carboxylic, phenolic and etheric groups. The stability of pristine and functionalized mesoporous carbons dispersed in water was analyzed by multiple light scattering and laser diffraction methods. Additionally, the zeta potential of mesoporous carbons suspensions was measured. Particle–particle interactions, particle size and their density determined the sedimentation behavior of materials in aqueous solution. Non-modified CKIT-6 and CSBA-15 exhibited higher transmission level than the oxidized materials. Higher transmission intensity is related to more intense sedimentation. The results indicate that the dispersion of functionalized CKIT-6 and CSBA-15 in a liquid phase is much more stable than that of non-modified mesoporous carbon materials. In contrast, for the carbon samples obtained by the soft template method, the modification did not produce significant changes in the intensity of back-scattered light.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide