| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145822 | Chemical Engineering Journal | 2016 | 13 Pages |
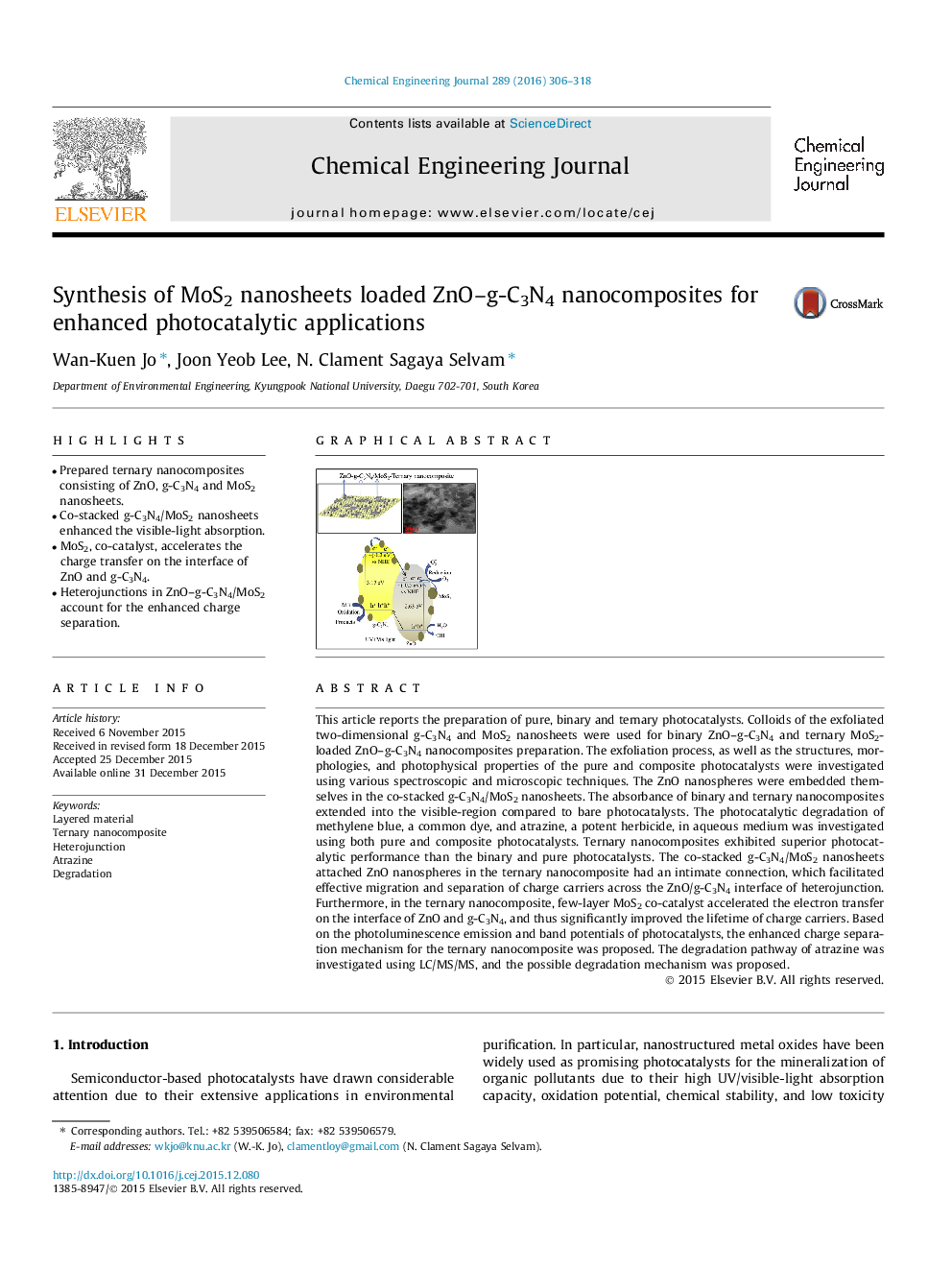
•Prepared ternary nanocomposites consisting of ZnO, g-C3N4 and MoS2 nanosheets.•Co-stacked g-C3N4/MoS2 nanosheets enhanced the visible-light absorption.•MoS2, co-catalyst, accelerates the charge transfer on the interface of ZnO and g-C3N4.•Heterojunctions in ZnO–g-C3N4/MoS2 account for the enhanced charge separation.
This article reports the preparation of pure, binary and ternary photocatalysts. Colloids of the exfoliated two-dimensional g-C3N4 and MoS2 nanosheets were used for binary ZnO–g-C3N4 and ternary MoS2-loaded ZnO–g-C3N4 nanocomposites preparation. The exfoliation process, as well as the structures, morphologies, and photophysical properties of the pure and composite photocatalysts were investigated using various spectroscopic and microscopic techniques. The ZnO nanospheres were embedded themselves in the co-stacked g-C3N4/MoS2 nanosheets. The absorbance of binary and ternary nanocomposites extended into the visible-region compared to bare photocatalysts. The photocatalytic degradation of methylene blue, a common dye, and atrazine, a potent herbicide, in aqueous medium was investigated using both pure and composite photocatalysts. Ternary nanocomposites exhibited superior photocatalytic performance than the binary and pure photocatalysts. The co-stacked g-C3N4/MoS2 nanosheets attached ZnO nanospheres in the ternary nanocomposite had an intimate connection, which facilitated effective migration and separation of charge carriers across the ZnO/g-C3N4 interface of heterojunction. Furthermore, in the ternary nanocomposite, few-layer MoS2 co-catalyst accelerated the electron transfer on the interface of ZnO and g-C3N4, and thus significantly improved the lifetime of charge carriers. Based on the photoluminescence emission and band potentials of photocatalysts, the enhanced charge separation mechanism for the ternary nanocomposite was proposed. The degradation pathway of atrazine was investigated using LC/MS/MS, and the possible degradation mechanism was proposed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide