| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145826 | Chemical Engineering Journal | 2016 | 7 Pages |
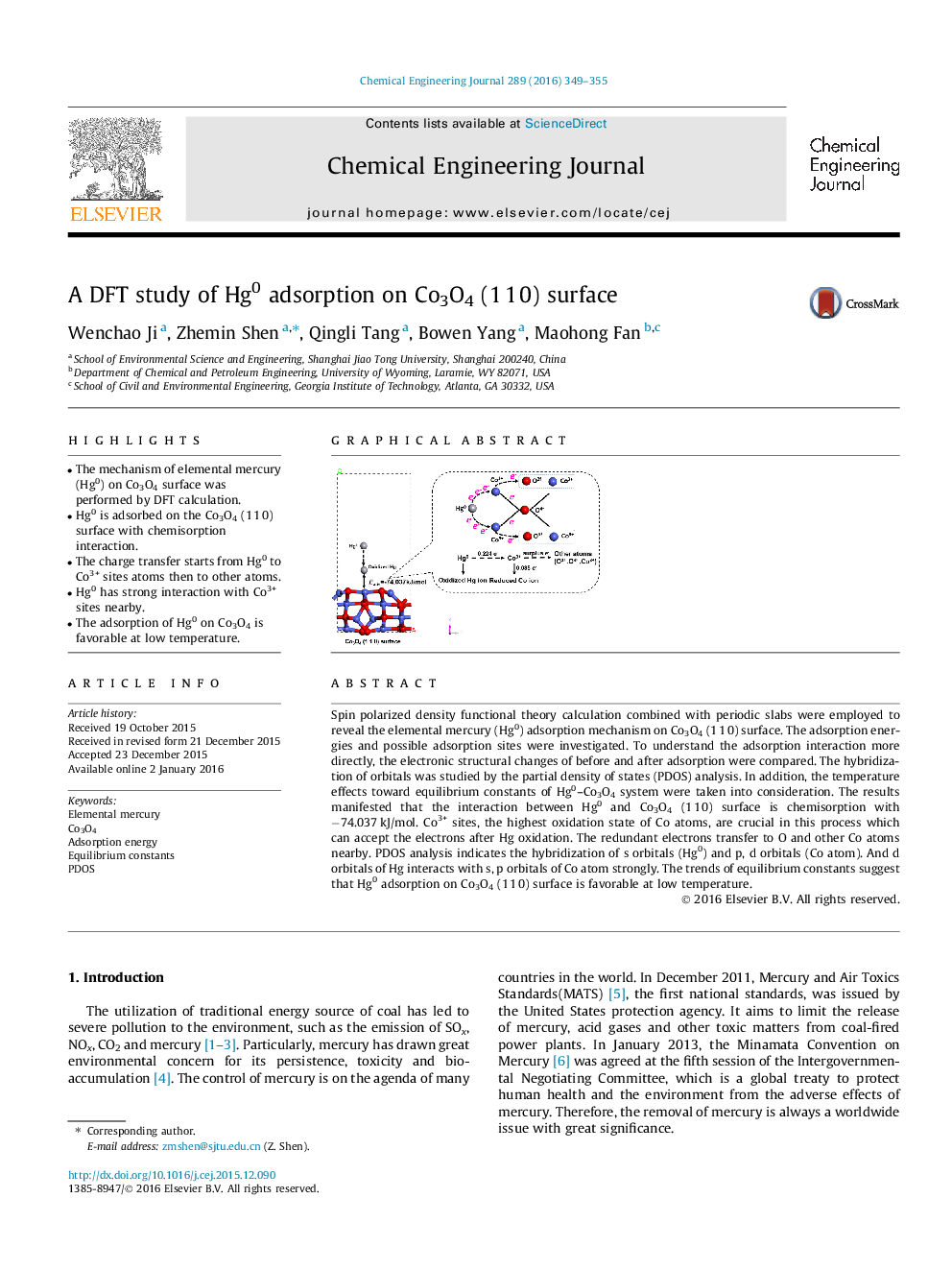
•The mechanism of elemental mercury (Hg0) on Co3O4 surface was performed by DFT calculation.•Hg0 is adsorbed on the Co3O4 (1 1 0) surface with chemisorption interaction.•The charge transfer starts from Hg0 to Co3+ sites atoms then to other atoms.•Hg0 has strong interaction with Co3+ sites nearby.•The adsorption of Hg0 on Co3O4 is favorable at low temperature.
Spin polarized density functional theory calculation combined with periodic slabs were employed to reveal the elemental mercury (Hg0) adsorption mechanism on Co3O4 (1 1 0) surface. The adsorption energies and possible adsorption sites were investigated. To understand the adsorption interaction more directly, the electronic structural changes of before and after adsorption were compared. The hybridization of orbitals was studied by the partial density of states (PDOS) analysis. In addition, the temperature effects toward equilibrium constants of Hg0–Co3O4 system were taken into consideration. The results manifested that the interaction between Hg0 and Co3O4 (1 1 0) surface is chemisorption with −74.037 kJ/mol. Co3+ sites, the highest oxidation state of Co atoms, are crucial in this process which can accept the electrons after Hg oxidation. The redundant electrons transfer to O and other Co atoms nearby. PDOS analysis indicates the hybridization of s orbitals (Hg0) and p, d orbitals (Co atom). And d orbitals of Hg interacts with s, p orbitals of Co atom strongly. The trends of equilibrium constants suggest that Hg0 adsorption on Co3O4 (1 1 0) surface is favorable at low temperature.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide