| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145843 | Chemical Engineering Journal | 2016 | 9 Pages |
•A two-compartment electrolytic cell was designed to reduce bromate.•Ti electrode was oxidized during electrochemically induced pitting corrosion.•Bromate was effectively reduced to bromide by reactive Ti species.•Pseudo-first-order model described the experimental data well.•The electrochemical mechanism of bromate reduction was proposed.
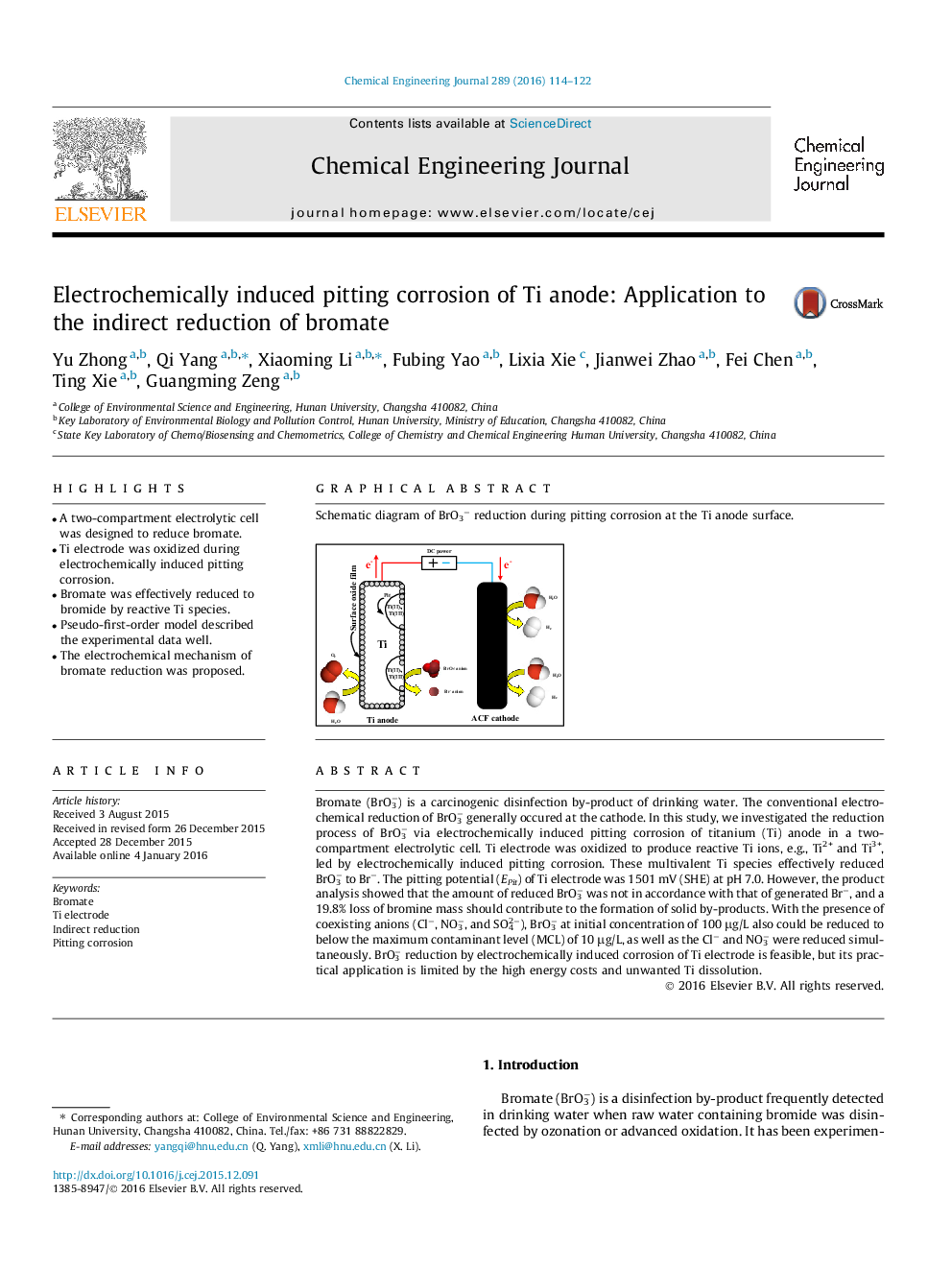
Bromate (BrO3−) is a carcinogenic disinfection by-product of drinking water. The conventional electrochemical reduction of BrO3− generally occured at the cathode. In this study, we investigated the reduction process of BrO3− via electrochemically induced pitting corrosion of titanium (Ti) anode in a two-compartment electrolytic cell. Ti electrode was oxidized to produce reactive Ti ions, e.g., Ti2+ and Ti3+, led by electrochemically induced pitting corrosion. These multivalent Ti species effectively reduced BrO3− to Br−. The pitting potential (EPit) of Ti electrode was 1501 mV (SHE) at pH 7.0. However, the product analysis showed that the amount of reduced BrO3− was not in accordance with that of generated Br−, and a 19.8% loss of bromine mass should contribute to the formation of solid by-products. With the presence of coexisting anions (Cl−, NO3−, and SO42−), BrO3− at initial concentration of 100 μg/L also could be reduced to below the maximum contaminant level (MCL) of 10 μg/L, as well as the Cl− and NO3− were reduced simultaneously. BrO3− reduction by electrochemically induced corrosion of Ti electrode is feasible, but its practical application is limited by the high energy costs and unwanted Ti dissolution.
Graphical abstractSchematic diagram of BrO3− reduction during pitting corrosion at the Ti anode surface.Figure optionsDownload full-size imageDownload as PowerPoint slide