| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145883 | Chemical Engineering Journal | 2016 | 8 Pages |
•The recycling alum sludge as a new coagulant aid was used to enhance removal of HA.•Charge neutralization, entrapment and adsorption are dominant in dual-coagulants.•Addition of AHF improved flocs with large size and fractal dimension.•The regrowth of breakage flocs facilitated the further removal of the organic matters.•The regenerated flocs could be an adsorbent to further remove organic matter.
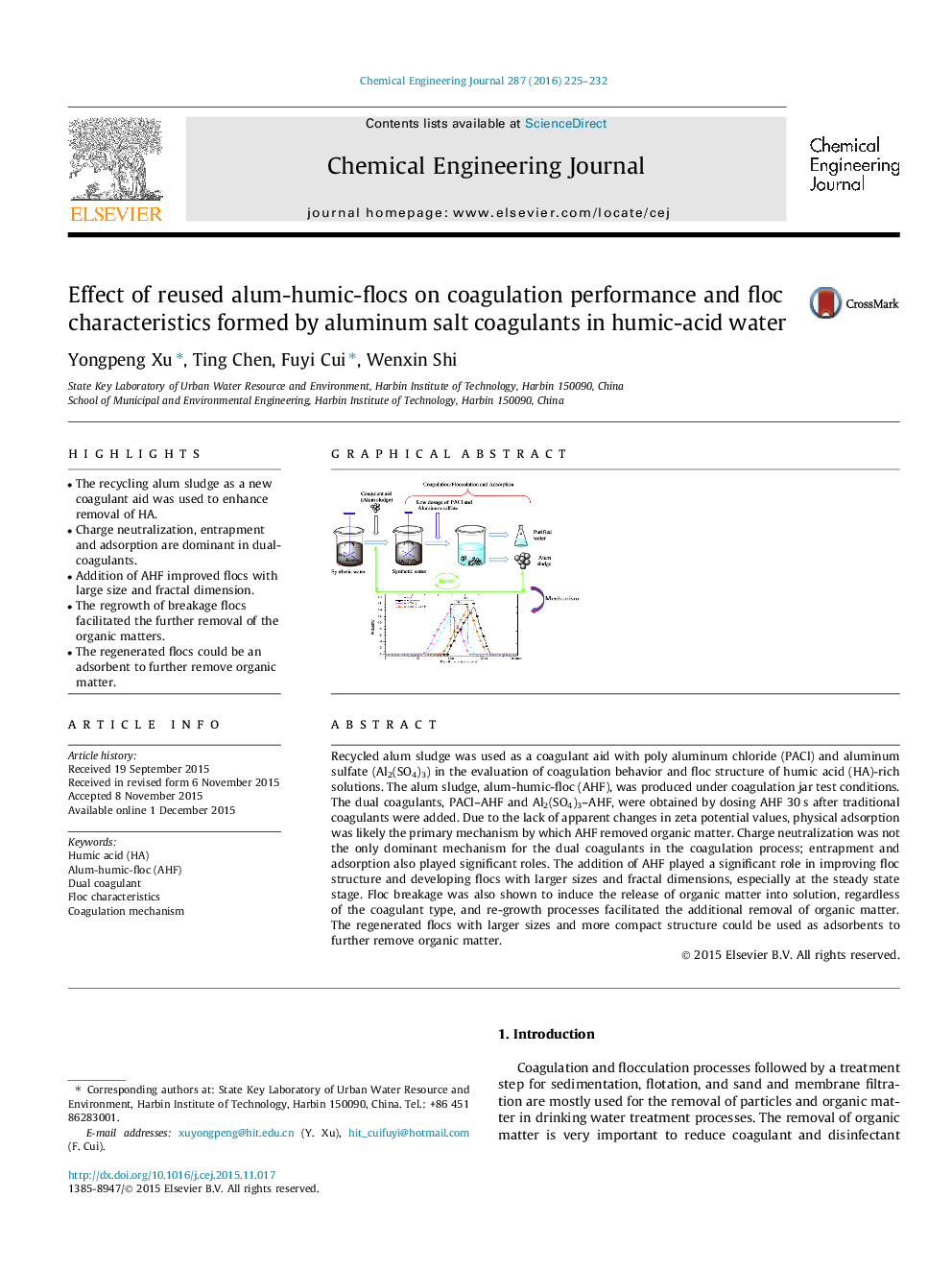
Recycled alum sludge was used as a coagulant aid with poly aluminum chloride (PACl) and aluminum sulfate (Al2(SO4)3) in the evaluation of coagulation behavior and floc structure of humic acid (HA)-rich solutions. The alum sludge, alum-humic-floc (AHF), was produced under coagulation jar test conditions. The dual coagulants, PACl–AHF and Al2(SO4)3–AHF, were obtained by dosing AHF 30 s after traditional coagulants were added. Due to the lack of apparent changes in zeta potential values, physical adsorption was likely the primary mechanism by which AHF removed organic matter. Charge neutralization was not the only dominant mechanism for the dual coagulants in the coagulation process; entrapment and adsorption also played significant roles. The addition of AHF played a significant role in improving floc structure and developing flocs with larger sizes and fractal dimensions, especially at the steady state stage. Floc breakage was also shown to induce the release of organic matter into solution, regardless of the coagulant type, and re-growth processes facilitated the additional removal of organic matter. The regenerated flocs with larger sizes and more compact structure could be used as adsorbents to further remove organic matter.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide