| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145899 | Chemical Engineering Journal | 2016 | 8 Pages |
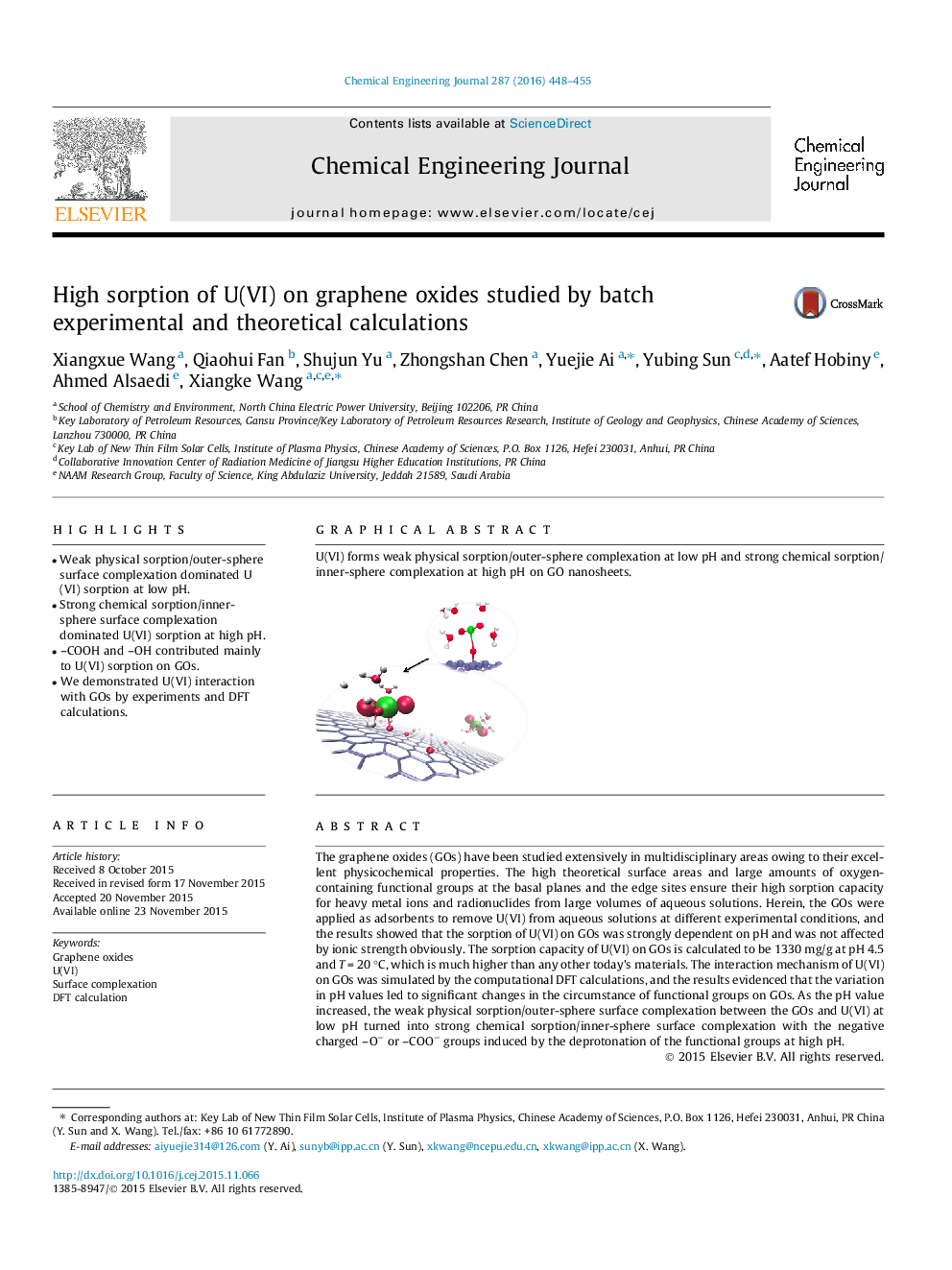
•Weak physical sorption/outer-sphere surface complexation dominated U(VI) sorption at low pH.•Strong chemical sorption/inner-sphere surface complexation dominated U(VI) sorption at high pH.•–COOH and –OH contributed mainly to U(VI) sorption on GOs.•We demonstrated U(VI) interaction with GOs by experiments and DFT calculations.
The graphene oxides (GOs) have been studied extensively in multidisciplinary areas owing to their excellent physicochemical properties. The high theoretical surface areas and large amounts of oxygen-containing functional groups at the basal planes and the edge sites ensure their high sorption capacity for heavy metal ions and radionuclides from large volumes of aqueous solutions. Herein, the GOs were applied as adsorbents to remove U(VI) from aqueous solutions at different experimental conditions, and the results showed that the sorption of U(VI) on GOs was strongly dependent on pH and was not affected by ionic strength obviously. The sorption capacity of U(VI) on GOs is calculated to be 1330 mg/g at pH 4.5 and T = 20 °C, which is much higher than any other today’s materials. The interaction mechanism of U(VI) on GOs was simulated by the computational DFT calculations, and the results evidenced that the variation in pH values led to significant changes in the circumstance of functional groups on GOs. As the pH value increased, the weak physical sorption/outer-sphere surface complexation between the GOs and U(VI) at low pH turned into strong chemical sorption/inner-sphere surface complexation with the negative charged –O− or –COO− groups induced by the deprotonation of the functional groups at high pH.
Graphical abstractU(VI) forms weak physical sorption/outer-sphere complexation at low pH and strong chemical sorption/inner-sphere complexation at high pH on GO nanosheets.Figure optionsDownload full-size imageDownload as PowerPoint slide