| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 145937 | Chemical Engineering Journal | 2016 | 8 Pages |
•Urea–formaldehyde in the presence of MMT is effective to obtain fertilizer.•Fertilizer nanocomposites were produced by cold plastic extrusion.•Fertilizer nanocomposites presented good mechanical resistance.•Efficient results were found in water-solubility and soil incubation tests.•Intermediate urea–paraformaldehyde molar ratio showed most efficient results.
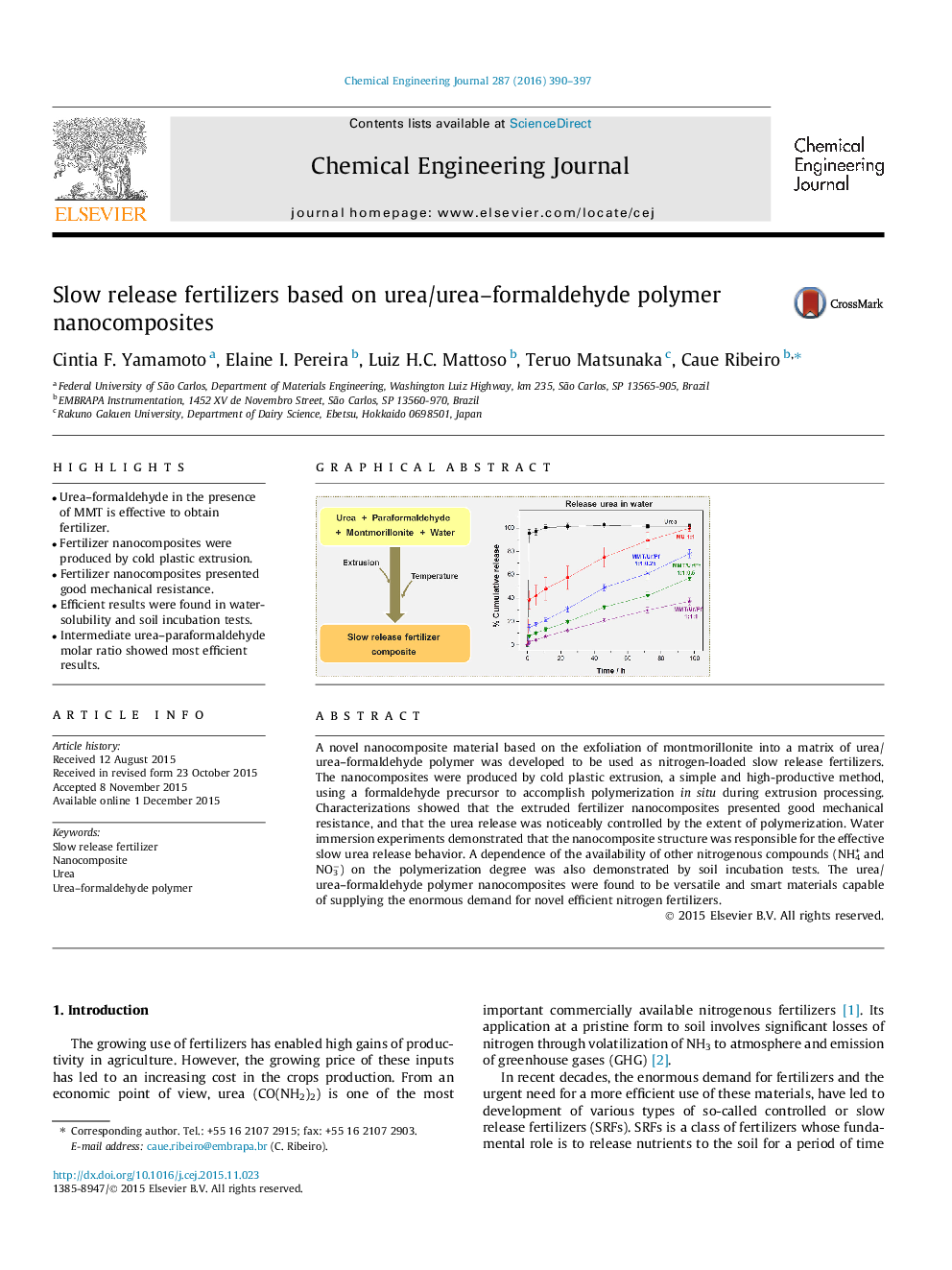
A novel nanocomposite material based on the exfoliation of montmorillonite into a matrix of urea/urea–formaldehyde polymer was developed to be used as nitrogen-loaded slow release fertilizers. The nanocomposites were produced by cold plastic extrusion, a simple and high-productive method, using a formaldehyde precursor to accomplish polymerization in situ during extrusion processing. Characterizations showed that the extruded fertilizer nanocomposites presented good mechanical resistance, and that the urea release was noticeably controlled by the extent of polymerization. Water immersion experiments demonstrated that the nanocomposite structure was responsible for the effective slow urea release behavior. A dependence of the availability of other nitrogenous compounds (NH4+ and NO3−) on the polymerization degree was also demonstrated by soil incubation tests. The urea/urea–formaldehyde polymer nanocomposites were found to be versatile and smart materials capable of supplying the enormous demand for novel efficient nitrogen fertilizers.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide