| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146015 | Chemical Engineering Journal | 2016 | 8 Pages |
•UV radiation is not effective to degrade cytarabine at both acidic and neutral pHs.•Cytarabine degradation was more effective with the UV/S2O82− process.•ΔH values were +46.4 for H2O2 → 2HO and +50.8 kcal mol−1 for S2O82− → 2SO4.•Cytosine was the main degradation byproduct detected with all processes studied.•The influence of radical promoters, pH, type of water, and anions was studied.
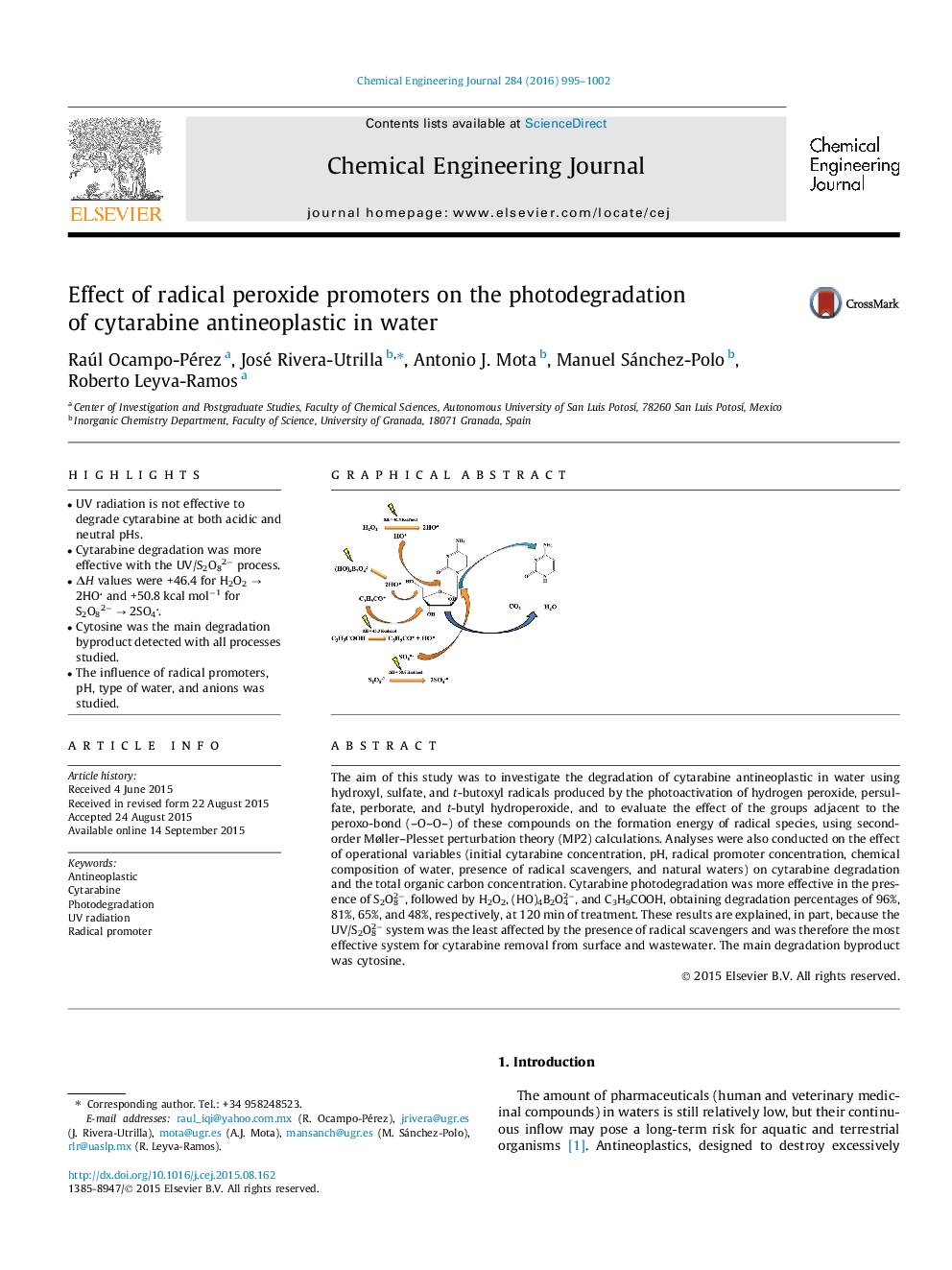
The aim of this study was to investigate the degradation of cytarabine antineoplastic in water using hydroxyl, sulfate, and t-butoxyl radicals produced by the photoactivation of hydrogen peroxide, persulfate, perborate, and t-butyl hydroperoxide, and to evaluate the effect of the groups adjacent to the peroxo-bond (–O–O–) of these compounds on the formation energy of radical species, using second-order Møller–Plesset perturbation theory (MP2) calculations. Analyses were also conducted on the effect of operational variables (initial cytarabine concentration, pH, radical promoter concentration, chemical composition of water, presence of radical scavengers, and natural waters) on cytarabine degradation and the total organic carbon concentration. Cytarabine photodegradation was more effective in the presence of S2O82−, followed by H2O2, (HO)4B2O42−, and C3H9COOH, obtaining degradation percentages of 96%, 81%, 65%, and 48%, respectively, at 120 min of treatment. These results are explained, in part, because the UV/S2O82− system was the least affected by the presence of radical scavengers and was therefore the most effective system for cytarabine removal from surface and wastewater. The main degradation byproduct was cytosine.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide