| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146037 | Chemical Engineering Journal | 2016 | 11 Pages |
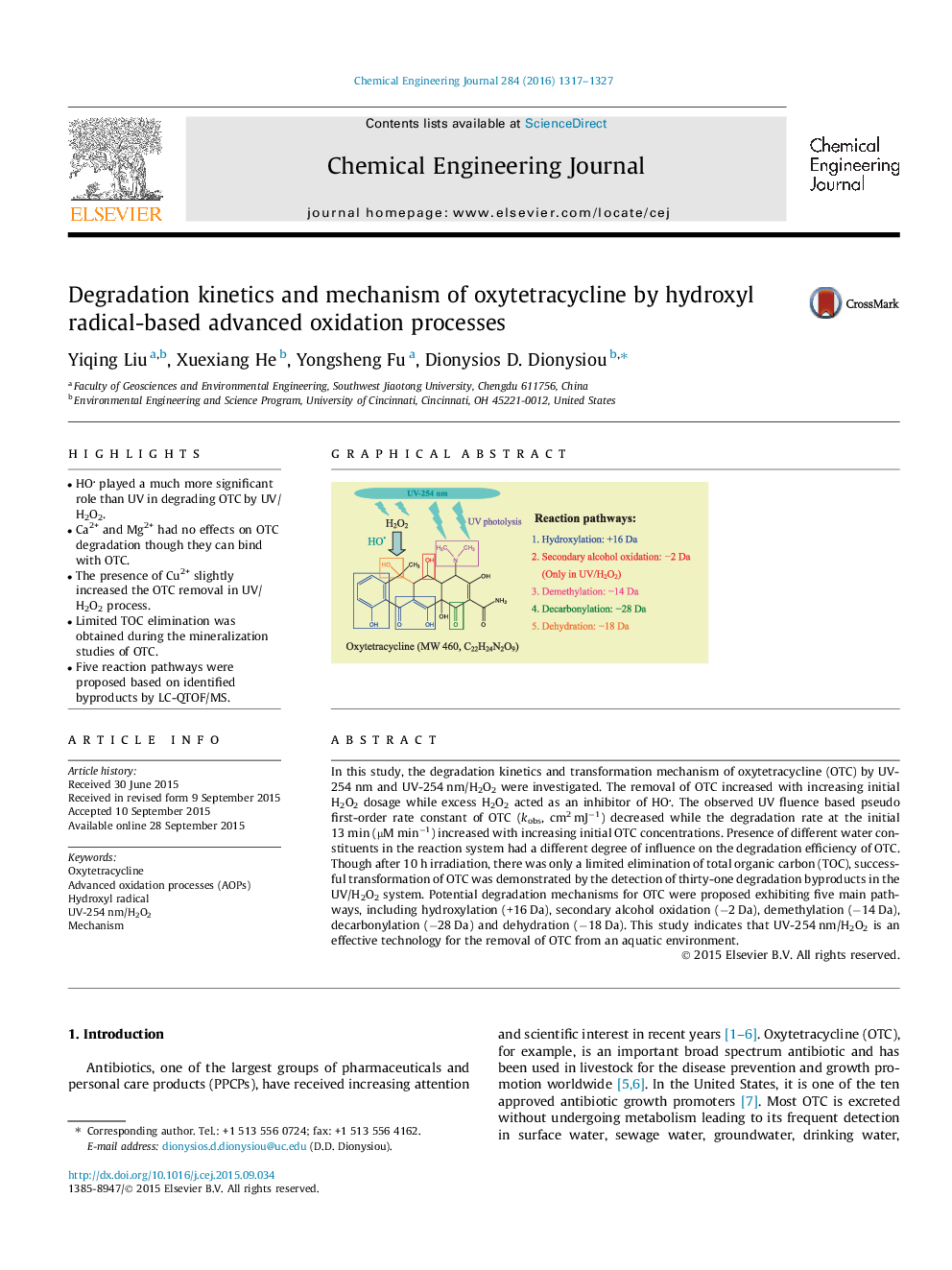
•HO played a much more significant role than UV in degrading OTC by UV/H2O2.•Ca2+ and Mg2+ had no effects on OTC degradation though they can bind with OTC.•The presence of Cu2+ slightly increased the OTC removal in UV/H2O2 process.•Limited TOC elimination was obtained during the mineralization studies of OTC.•Five reaction pathways were proposed based on identified byproducts by LC-QTOF/MS.
In this study, the degradation kinetics and transformation mechanism of oxytetracycline (OTC) by UV-254 nm and UV-254 nm/H2O2 were investigated. The removal of OTC increased with increasing initial H2O2 dosage while excess H2O2 acted as an inhibitor of HO. The observed UV fluence based pseudo first-order rate constant of OTC (kobs, cm2 mJ−1) decreased while the degradation rate at the initial 13 min (µM min−1) increased with increasing initial OTC concentrations. Presence of different water constituents in the reaction system had a different degree of influence on the degradation efficiency of OTC. Though after 10 h irradiation, there was only a limited elimination of total organic carbon (TOC), successful transformation of OTC was demonstrated by the detection of thirty-one degradation byproducts in the UV/H2O2 system. Potential degradation mechanisms for OTC were proposed exhibiting five main pathways, including hydroxylation (+16 Da), secondary alcohol oxidation (−2 Da), demethylation (−14 Da), decarbonylation (−28 Da) and dehydration (−18 Da). This study indicates that UV-254 nm/H2O2 is an effective technology for the removal of OTC from an aquatic environment.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide