| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146232 | Chemical Engineering Journal | 2015 | 12 Pages |
•Fe3O4 and CNCs were used to stabilize a Pickering double w1/o/w2 emulsion and the HM-IMs were prepared successfully.•The HM-IMs possessed well-designed multihollow structure.•The HM-IMs presented good magnetic sensitivity and thermal stability.•The HM-IMs exhibited satisfying selective adsorption capability for BF.
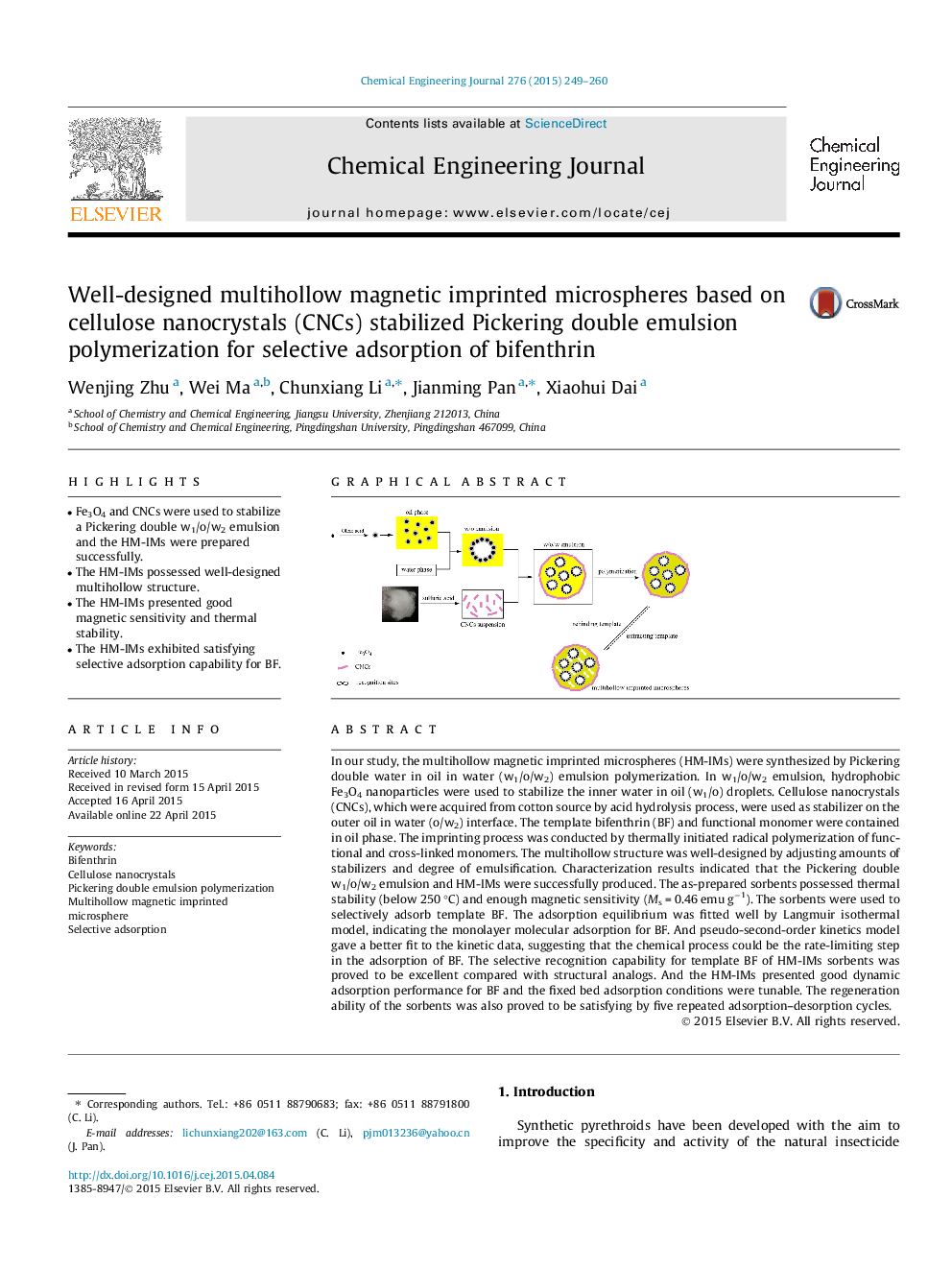
In our study, the multihollow magnetic imprinted microspheres (HM-IMs) were synthesized by Pickering double water in oil in water (w1/o/w2) emulsion polymerization. In w1/o/w2 emulsion, hydrophobic Fe3O4 nanoparticles were used to stabilize the inner water in oil (w1/o) droplets. Cellulose nanocrystals (CNCs), which were acquired from cotton source by acid hydrolysis process, were used as stabilizer on the outer oil in water (o/w2) interface. The template bifenthrin (BF) and functional monomer were contained in oil phase. The imprinting process was conducted by thermally initiated radical polymerization of functional and cross-linked monomers. The multihollow structure was well-designed by adjusting amounts of stabilizers and degree of emulsification. Characterization results indicated that the Pickering double w1/o/w2 emulsion and HM-IMs were successfully produced. The as-prepared sorbents possessed thermal stability (below 250 °C) and enough magnetic sensitivity (Ms = 0.46 emu g−1). The sorbents were used to selectively adsorb template BF. The adsorption equilibrium was fitted well by Langmuir isothermal model, indicating the monolayer molecular adsorption for BF. And pseudo-second-order kinetics model gave a better fit to the kinetic data, suggesting that the chemical process could be the rate-limiting step in the adsorption of BF. The selective recognition capability for template BF of HM-IMs sorbents was proved to be excellent compared with structural analogs. And the HM-IMs presented good dynamic adsorption performance for BF and the fixed bed adsorption conditions were tunable. The regeneration ability of the sorbents was also proved to be satisfying by five repeated adsorption–desorption cycles.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide