| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146247 | Chemical Engineering Journal | 2015 | 10 Pages |
•Cellulose hydrolysis was modeled and analyzed in pressurized water changing P and T.•Overall cellulose hydrolysis kinetic was not affected by pressure.•Glucose and fructose reaction kinetic were highly modified by changing P and T.•The selectivity of biomass hydrolysis can be set to the desired value by choosing P and T.
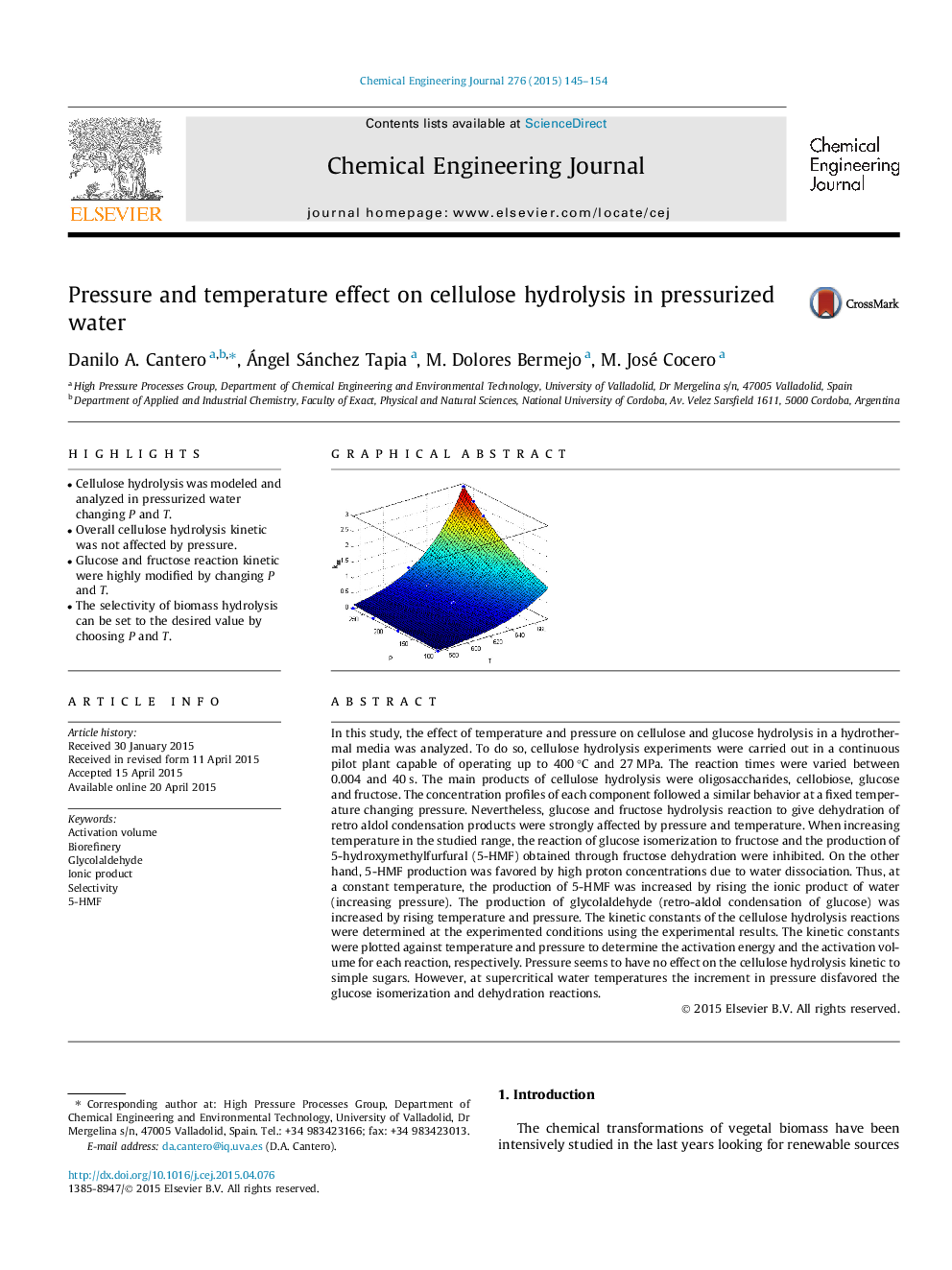
In this study, the effect of temperature and pressure on cellulose and glucose hydrolysis in a hydrothermal media was analyzed. To do so, cellulose hydrolysis experiments were carried out in a continuous pilot plant capable of operating up to 400 °C and 27 MPa. The reaction times were varied between 0.004 and 40 s. The main products of cellulose hydrolysis were oligosaccharides, cellobiose, glucose and fructose. The concentration profiles of each component followed a similar behavior at a fixed temperature changing pressure. Nevertheless, glucose and fructose hydrolysis reaction to give dehydration of retro aldol condensation products were strongly affected by pressure and temperature. When increasing temperature in the studied range, the reaction of glucose isomerization to fructose and the production of 5-hydroxymethylfurfural (5-HMF) obtained through fructose dehydration were inhibited. On the other hand, 5-HMF production was favored by high proton concentrations due to water dissociation. Thus, at a constant temperature, the production of 5-HMF was increased by rising the ionic product of water (increasing pressure). The production of glycolaldehyde (retro-aldol condensation of glucose) was increased by rising temperature and pressure. The kinetic constants of the cellulose hydrolysis reactions were determined at the experimented conditions using the experimental results. The kinetic constants were plotted against temperature and pressure to determine the activation energy and the activation volume for each reaction, respectively. Pressure seems to have no effect on the cellulose hydrolysis kinetic to simple sugars. However, at supercritical water temperatures the increment in pressure disfavored the glucose isomerization and dehydration reactions.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide