| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146272 | Chemical Engineering Journal | 2015 | 9 Pages |
•Removal of Cs-137 by an isotope dilution–precipitate flotation was studied.•Sodium tetraphenylborate precipitating agent was used with SLS and CPC surfactants.•Lower remaining Cs-137 activity values than the permissible value are obtained.•CsTPB precipitate was efficiently separated within half a minute.•The removal mechanism of cesium by SLS and CPC was proposed at different pH values.
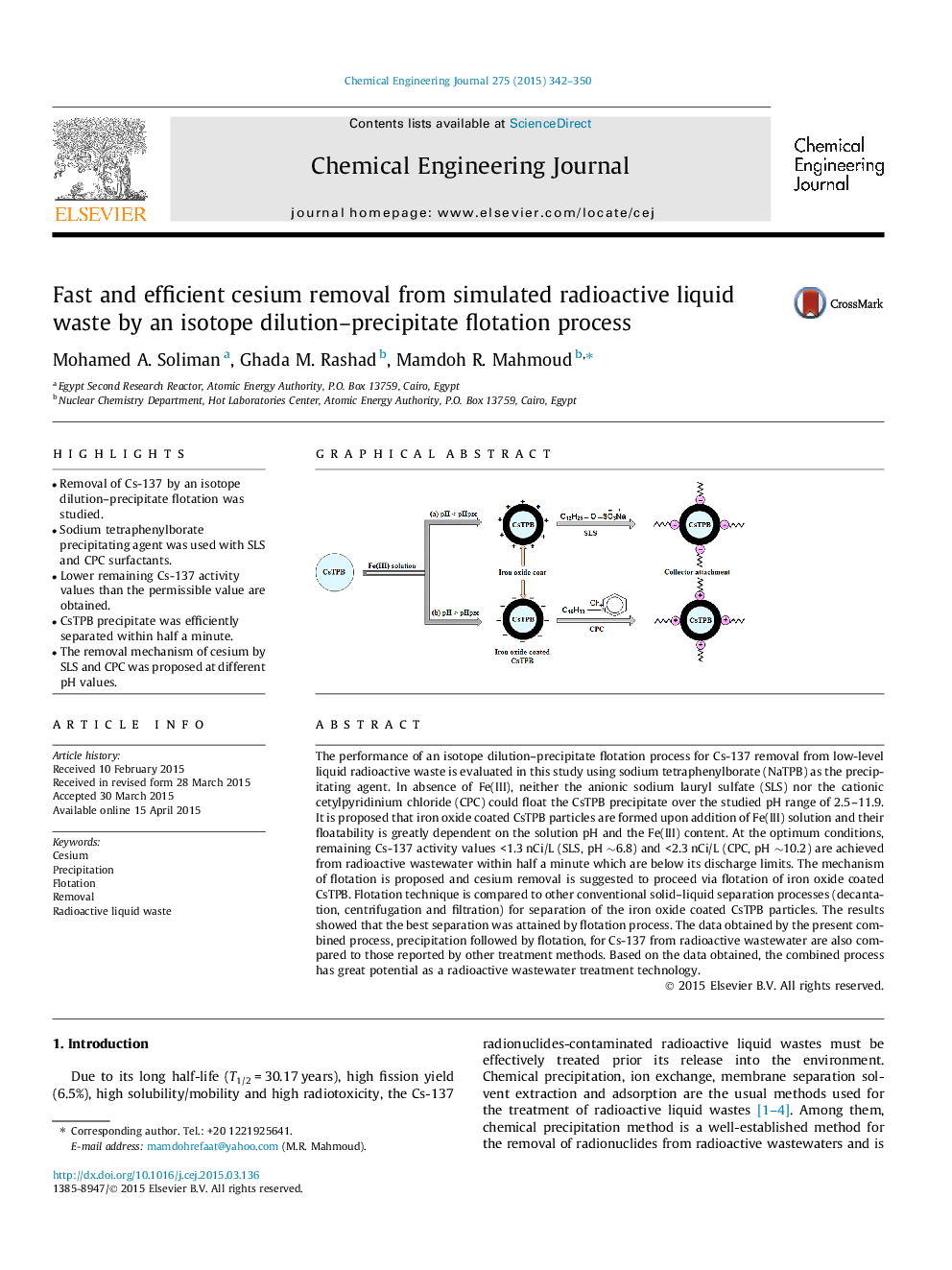
The performance of an isotope dilution–precipitate flotation process for Cs-137 removal from low-level liquid radioactive waste is evaluated in this study using sodium tetraphenylborate (NaTPB) as the precipitating agent. In absence of Fe(III), neither the anionic sodium lauryl sulfate (SLS) nor the cationic cetylpyridinium chloride (CPC) could float the CsTPB precipitate over the studied pH range of 2.5–11.9. It is proposed that iron oxide coated CsTPB particles are formed upon addition of Fe(III) solution and their floatability is greatly dependent on the solution pH and the Fe(III) content. At the optimum conditions, remaining Cs-137 activity values <1.3 nCi/L (SLS, pH ∼6.8) and <2.3 nCi/L (CPC, pH ∼10.2) are achieved from radioactive wastewater within half a minute which are below its discharge limits. The mechanism of flotation is proposed and cesium removal is suggested to proceed via flotation of iron oxide coated CsTPB. Flotation technique is compared to other conventional solid–liquid separation processes (decantation, centrifugation and filtration) for separation of the iron oxide coated CsTPB particles. The results showed that the best separation was attained by flotation process. The data obtained by the present combined process, precipitation followed by flotation, for Cs-137 from radioactive wastewater are also compared to those reported by other treatment methods. Based on the data obtained, the combined process has great potential as a radioactive wastewater treatment technology.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide