| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146342 | Chemical Engineering Journal | 2015 | 8 Pages |
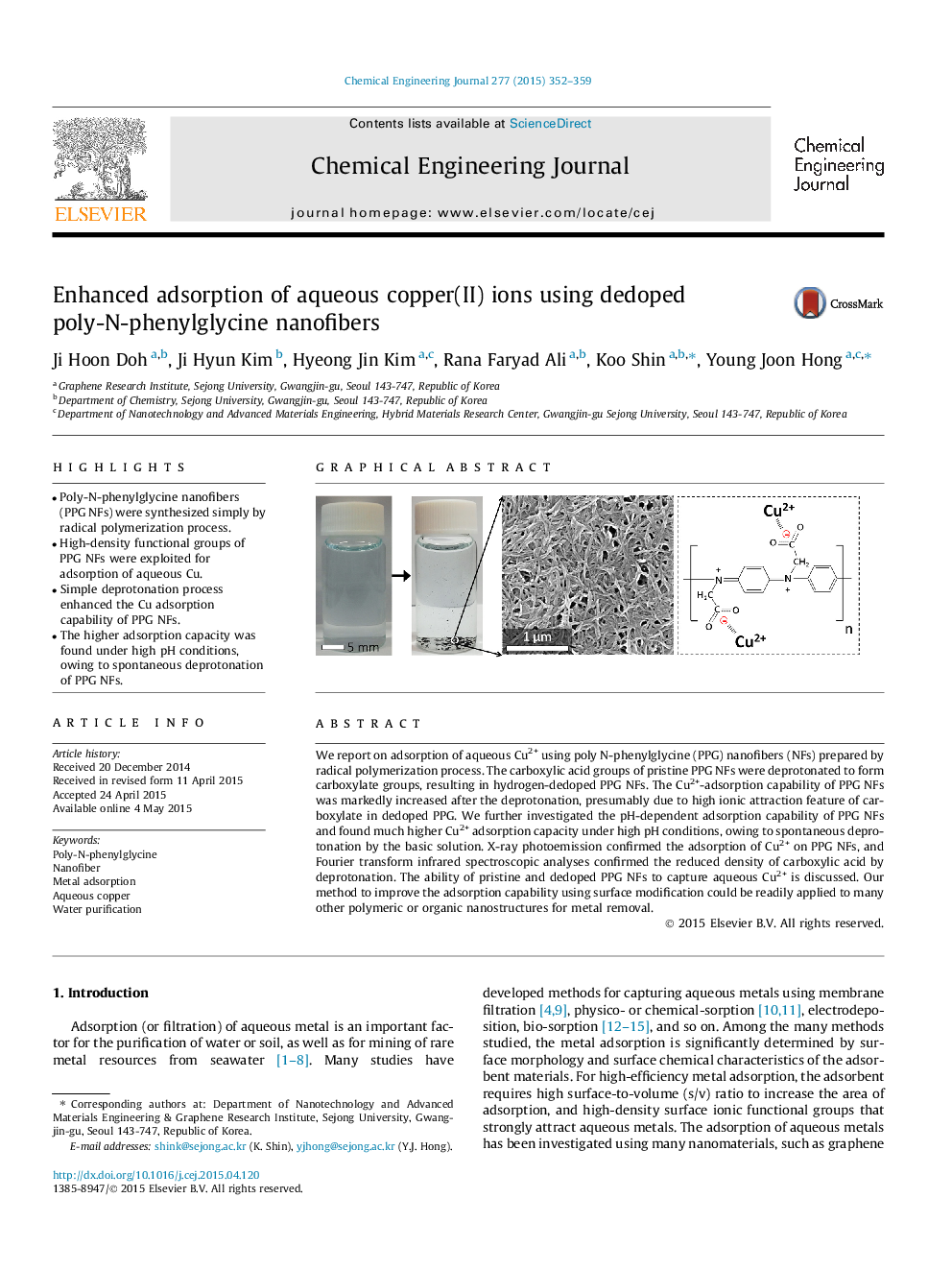
•Poly-N-phenylglycine nanofibers (PPG NFs) were synthesized simply by radical polymerization process.•High-density functional groups of PPG NFs were exploited for adsorption of aqueous Cu.•Simple deprotonation process enhanced the Cu adsorption capability of PPG NFs.•The higher adsorption capacity was found under high pH conditions, owing to spontaneous deprotonation of PPG NFs.
We report on adsorption of aqueous Cu2+ using poly N-phenylglycine (PPG) nanofibers (NFs) prepared by radical polymerization process. The carboxylic acid groups of pristine PPG NFs were deprotonated to form carboxylate groups, resulting in hydrogen-dedoped PPG NFs. The Cu2+-adsorption capability of PPG NFs was markedly increased after the deprotonation, presumably due to high ionic attraction feature of carboxylate in dedoped PPG. We further investigated the pH-dependent adsorption capability of PPG NFs and found much higher Cu2+ adsorption capacity under high pH conditions, owing to spontaneous deprotonation by the basic solution. X-ray photoemission confirmed the adsorption of Cu2+ on PPG NFs, and Fourier transform infrared spectroscopic analyses confirmed the reduced density of carboxylic acid by deprotonation. The ability of pristine and dedoped PPG NFs to capture aqueous Cu2+ is discussed. Our method to improve the adsorption capability using surface modification could be readily applied to many other polymeric or organic nanostructures for metal removal.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide