| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146347 | Chemical Engineering Journal | 2015 | 13 Pages |
•The effect of pH on the structure and performance in the synthesis of FeVO4 was studied.•FeVO4 synthesized at pH = 4.5 showed relatively homogeneous nanoparticles.•FeVO4 synthesized at pH = 4.5 contained the largest amount of oxygen vacancies.•The surface-enrichment of FeVO4 played a key role in its improved SCR activity.
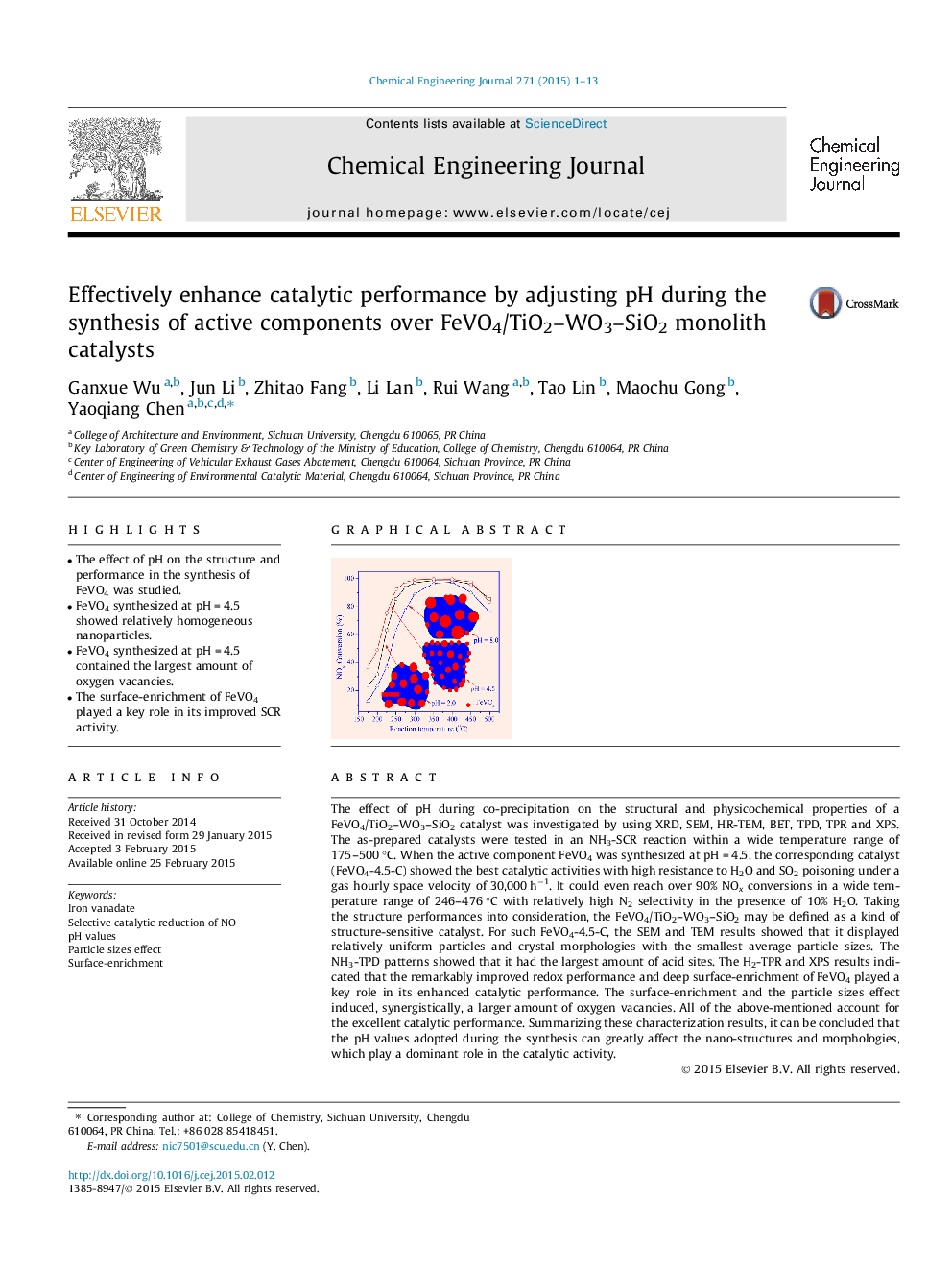
The effect of pH during co-precipitation on the structural and physicochemical properties of a FeVO4/TiO2–WO3–SiO2 catalyst was investigated by using XRD, SEM, HR-TEM, BET, TPD, TPR and XPS. The as-prepared catalysts were tested in an NH3-SCR reaction within a wide temperature range of 175–500 °C. When the active component FeVO4 was synthesized at pH = 4.5, the corresponding catalyst (FeVO4-4.5-C) showed the best catalytic activities with high resistance to H2O and SO2 poisoning under a gas hourly space velocity of 30,000 h−1. It could even reach over 90% NOx conversions in a wide temperature range of 246–476 °C with relatively high N2 selectivity in the presence of 10% H2O. Taking the structure performances into consideration, the FeVO4/TiO2–WO3–SiO2 may be defined as a kind of structure-sensitive catalyst. For such FeVO4-4.5-C, the SEM and TEM results showed that it displayed relatively uniform particles and crystal morphologies with the smallest average particle sizes. The NH3-TPD patterns showed that it had the largest amount of acid sites. The H2-TPR and XPS results indicated that the remarkably improved redox performance and deep surface-enrichment of FeVO4 played a key role in its enhanced catalytic performance. The surface-enrichment and the particle sizes effect induced, synergistically, a larger amount of oxygen vacancies. All of the above-mentioned account for the excellent catalytic performance. Summarizing these characterization results, it can be concluded that the pH values adopted during the synthesis can greatly affect the nano-structures and morphologies, which play a dominant role in the catalytic activity.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide