| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 146454 | Chemical Engineering Journal | 2015 | 8 Pages |
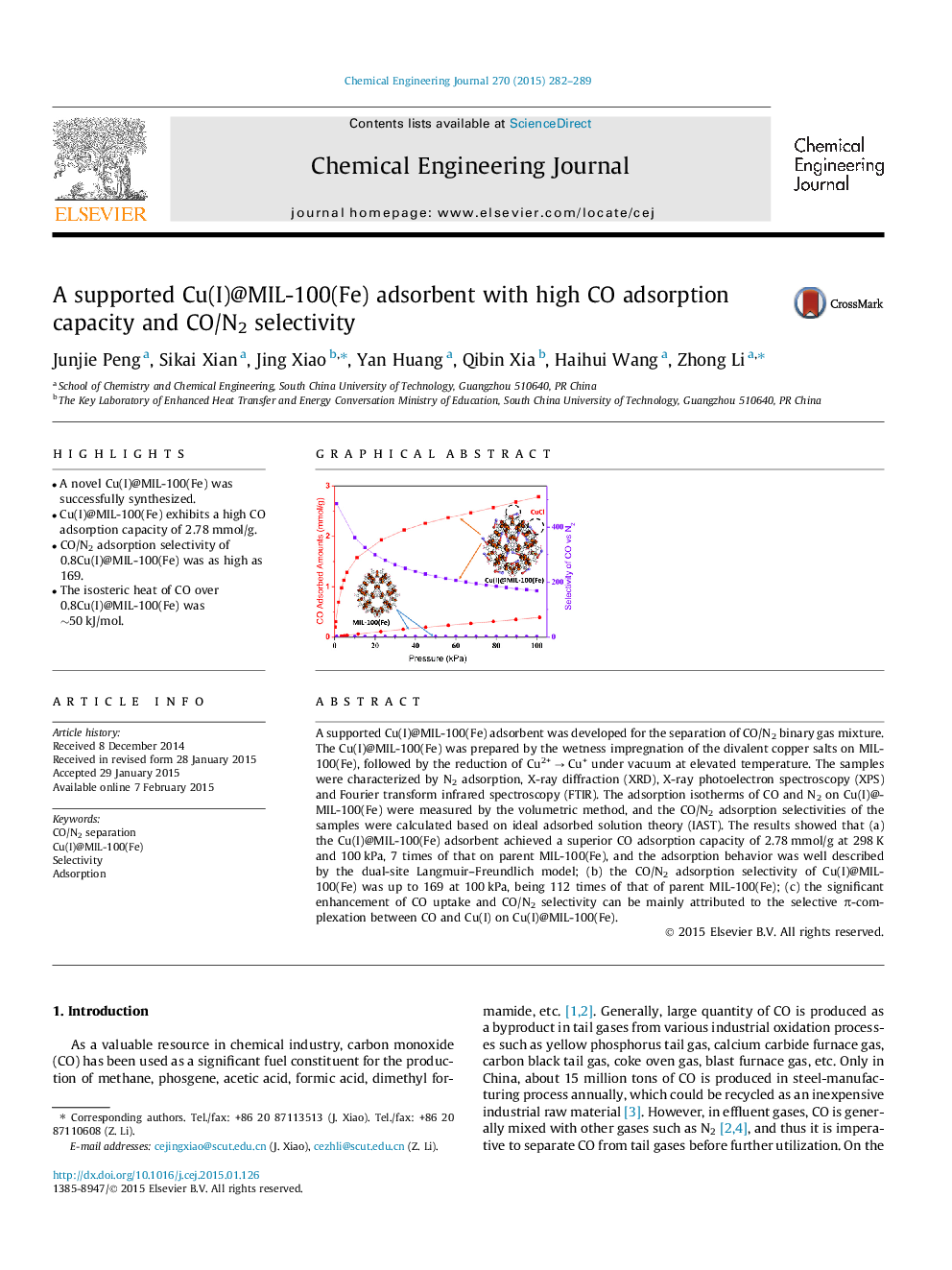
•A novel Cu(I)@MIL-100(Fe) was successfully synthesized.•Cu(I)@MIL-100(Fe) exhibits a high CO adsorption capacity of 2.78 mmol/g.•CO/N2 adsorption selectivity of 0.8Cu(I)@MIL-100(Fe) was as high as 169.•The isosteric heat of CO over 0.8Cu(I)@MIL-100(Fe) was ∼50 kJ/mol.
A supported Cu(I)@MIL-100(Fe) adsorbent was developed for the separation of CO/N2 binary gas mixture. The Cu(I)@MIL-100(Fe) was prepared by the wetness impregnation of the divalent copper salts on MIL-100(Fe), followed by the reduction of Cu2+ → Cu+ under vacuum at elevated temperature. The samples were characterized by N2 adsorption, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR). The adsorption isotherms of CO and N2 on Cu(I)@MIL-100(Fe) were measured by the volumetric method, and the CO/N2 adsorption selectivities of the samples were calculated based on ideal adsorbed solution theory (IAST). The results showed that (a) the Cu(I)@MIL-100(Fe) adsorbent achieved a superior CO adsorption capacity of 2.78 mmol/g at 298 K and 100 kPa, 7 times of that on parent MIL-100(Fe), and the adsorption behavior was well described by the dual-site Langmuir–Freundlich model; (b) the CO/N2 adsorption selectivity of Cu(I)@MIL-100(Fe) was up to 169 at 100 kPa, being 112 times of that of parent MIL-100(Fe); (c) the significant enhancement of CO uptake and CO/N2 selectivity can be mainly attributed to the selective π-complexation between CO and Cu(I) on Cu(I)@MIL-100(Fe).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide